Chapter 18
The Gastrointestinal Tract
The gastrointestinal (GI) tract is an endoderm-derived structure, consisting of the esophagus, stomach, small intestine, and large intestine. Although the primary purpose of the GI tract is the digestion and absorption of food, it also has important secondary roles, including immune functions, elimination of waste products, and endocrine effects. As such, diseases of the GI tract are a major cause of morbidity and mortality in companion animals and include a wide variety of conditions such as infectious diseases, neoplasia and other mass lesions, motility disorders, congenital disorders, and effects of medications. In this chapter, we will focus on diseases and conditions of the GI tract that can be diagnosed via cytologic examination of various sample types. In addition, we will address sampling techniques, normal findings, and common or important diagnostic dilemmas, and we will also introduce important aspects of histopathology of the GI tract. Because infectious organisms are an important cause of GI disease and will be discussed throughout the chapter, a detailed description of the most common organisms, their location within the GI tract, and additional useful diagnostic testing may be found in Table 18-1.1–3
TABLE 18-1
Infectious Agents of the Gastrointestinal Tract
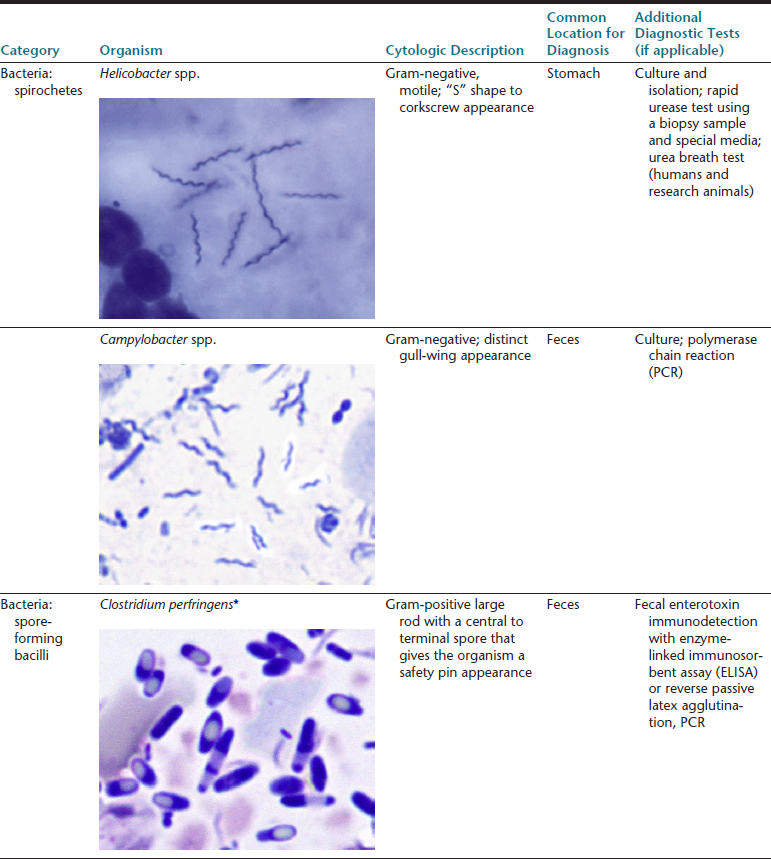
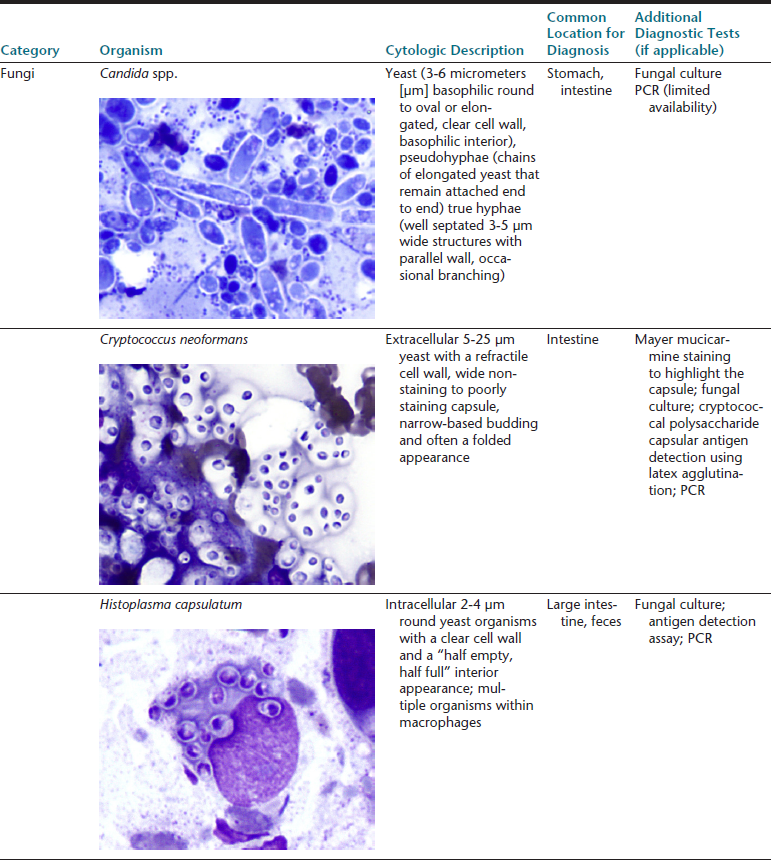
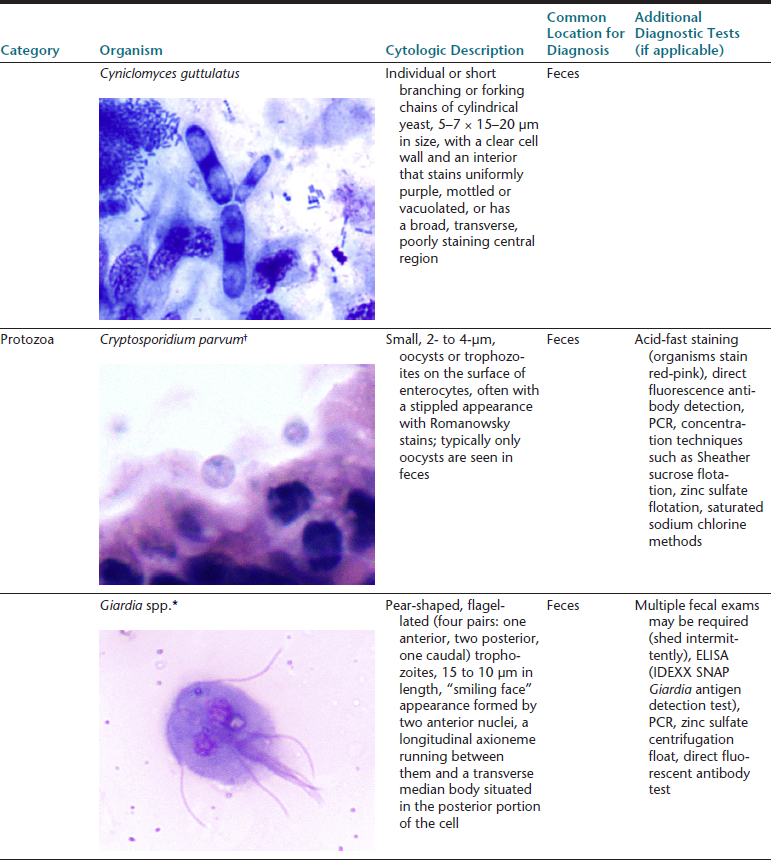
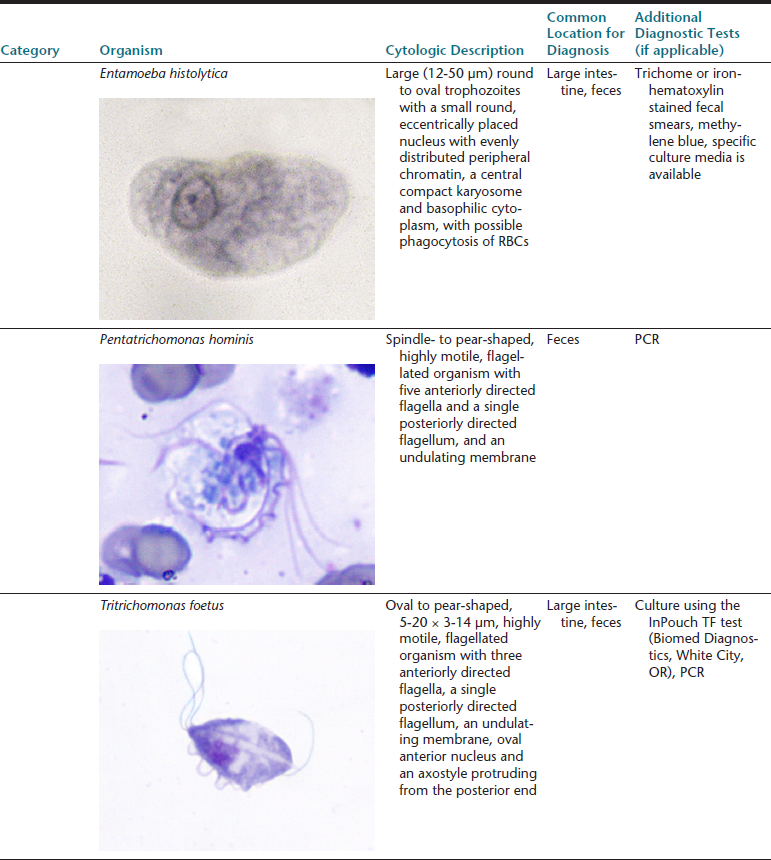
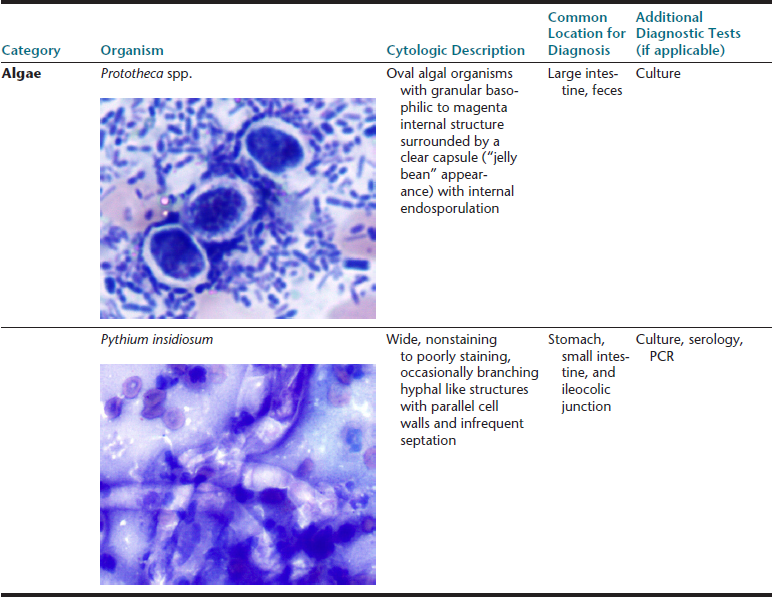
∗Image courtesy of Rick Cowell.
†Slide courtesy of Jody Gookin.
From Broussard JD: Optimal fecal assessment, Clin Tech Small Anim Pract 18:218, 2003; Greene CE: Infectious diseases of the dog and cat, ed 4, St. Louis, M, 2012, Saunders; Marks SL, Rankin SC, Byrne BA, et al: Enteropathogenic bacteria in dogs and cats: diagnosis, epidemiology, treatment, and control, J Vet Int Med 25:1195, 2011.
Sampling Techniques for the Gastrointestinal Tract
Ultrasound-Guided Sampling
Ultrasonography is commonly used to evaluate the intestinal tract in dogs and cats and is often used to obtain aspirate and tissue biopsy samples. Ultrasonographic evaluation is very helpful in aiding the diagnosis of GI diseases, particularly with infiltrative GI neoplasia; however, it is important to note that overlap exists between the ultrasonographic appearance of neoplastic and nonneoplastic diseases; therefore, ultrasonography is not entirely specific.4 Cytology from aspiration of the wall of the GI tract usually has low yield unless a significant lesion or increased wall thickness is present. Although ultrasound-guided aspirates or biopsies have the advantages of being less invasive and less expensive than obtaining a full-thickness biopsy, full-thickness biopsies remain the gold standard for differentiating certain inflammatory and neoplastic diseases such as intestinal lymphoma versus inflammatory bowel disease.
Endoscopy
Endoscopy is a well-established procedure for examining the GI tract and serves as an important alternative to exploratory surgery for direct examination of the tissues and anatomy. It is minimally invasive and allows visualization and sample collection of the luminal surface of the esophagus, stomach, proximal small intestine, and distal large intestine.5 Access to the remainder of the intestinal tract is limited during an endoscopic procedure. Endoscopic mucosal brushing and endoscopic biopsies are the main sample types that are collected during this procedure and predominantly evaluate the mucosal surface. Although these samples are useful for detecting surface inflammation and occasionally neoplasia, lesions that are deep to the mucosal surface may not be identified.
Laparoscopy and Abdominal Exploration
Laparoscopy is an operative procedure that is performed through a keyhole opening with a rigid endoscope. It allows for visual inspection of the organs and, in specialized settings, may allow for laparoscopy-assisted full-thickness biopsy of the GI tract. Abdominal exploration is a fairly invasive surgical procedure that is often used to obtain full-thickness biopsy samples of the GI tract. Although these procedures are more invasive, full-thickness biopsies in some cases will provide the best opportunity for obtaining a definitive diagnosis. In addition, the generally larger biopsy size obtained via these techniques may allow touch imprints of the tissue to be made prior to placing it in formalin. Cytologic evaluation of touch imprints may sometimes provide a preliminary or definitive diagnosis before histopathology results are available. Although care must be taken to preserve the integrity of the biopsy sample, it is important to gently wipe or blot the blood and serum off of the tissue before making the touch imprints to allow proper adhesion of cells to the slide. It is also possible to make touch imprints of ultrasound-guided or endoscopically obtained tissue biopsies, but the smaller size and greater fragility of these types of samples makes this more difficult.
Fecal Examination
Fecal testing is a common diagnostic procedure in the clinical evaluation of GI disease. Timing of sample collection and preparation is very important. Feces are altered after stool is passed and significant degeneration of cells and some organisms such as Giardia spp. or Tritrichomonas foetus can occur rapidly making identification increasingly difficult with time (Figure 18-1).1 With delayed processing of the fecal sample, some nematode eggs will release larvae, and bacterial overgrowth may occur. A fresh sample that can be processed immediately is best.
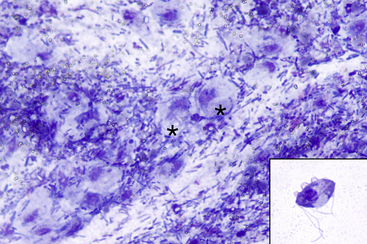
Figure 18-1 Feline fecal smear; degenerating Tritrichomonas foetus organisms.
Many degenerating organisms are present (two are indicated by asterisks [∗]) on a background of fecal material. The degenerating trophozoites are large round structures with round, dark, basophilic nuclei and an abundant amount of light basophilic cytoplasm. These could easily be mistaken for debris, degenerating tissue cells or histiocytes. Tritrichomonas degenerates quickly on exiting the body; thus, it is imperative that smears or wet mount preparations be made with fresh feces. The inset image is of a cytology preparation made from cultured T. foetus trophozoites. Typical morphologic features may be seen, including three anterior flagella, an undulating membrane, a longitudinal axostyle, an anterior nucleus, and a single posterior flagellum. (Modified Wright-Giemsa stain. Original magnification 50× objective. Inset: Wright-Giemsa stain. Original magnification 100× objective.). (Tritrichomonas culture courtesy of Katie Tolbert.)
Several collection techniques are used for either the luminal contents or the surface mucosa of the rectum. Defecated feces and feces obtained during a digital rectal examination or with a fecal loop represent the rectal lumen, whereas rectal lavage or rectal scrape samples are representative of the mucosal surface. If defecated feces are used, it is critical that they be fresh and not heavily contaminated with debris. Additional fecal diagnostics include fecal flotation, fecal sedimentation, and the Baermann technique; consultation of a veterinary parasitology text is recommended for additional information about these techniques.
When sampling the feces or rectal mucosa, lubricant gel should be used sparingly or avoided entirely, since the lubricant material could complicate evaluation of the cytologic specimen. Cytologically, lubricant has the appearance of thick extracellular magenta aggregates of material that may obscure the cells and organisms within the sample (Figure 18-2).
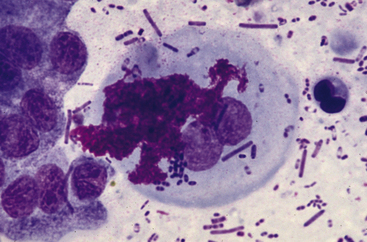
Figure 18-2 Fecal sample from a dog; abundant lubricant gel.
Few columnar epithelial cells are present at the left of the image with two squamous epithelial cells in the center. The morphologic characteristics of the squamous epithelial cells are partially obscured by bright pink–magenta granular to globular lubricant material. (Wright-Leishman stain.)
Direct Smears of Fecal Material
Direct smears from a luminal sample are made by spreading a small amount of feces thinly and evenly across the slide. The sample should not be heat-fixed; it is unnecessary and could cause significant damage to cells and organisms.1 After air-drying, the slide may be stained with a standard Romanowsky-type stain (e.g. Wright stain, Giemsa stain, rapid stains) and evaluated like any cytologic sample. If using a rapid stain (i.e., Diff-Quik), it is recommended to have a separate “dirty” staining station for fecal and ear cytology so as not to contaminate the station where “clean” cytology and hematology samples are stained.
Rectal Lavage
To perform a rectal lavage, the end of a lubricated red rubber catheter is inserted into the rectum and approximately 6 to 12 milliliters (mL) of saline is infused and aspirated multiple times until the sample has a mudlike appearance.1 Rectal lavage yields a small sample but allows the sample to be directly examined as an unstained wet mount preparation rather than as a stained dry fecal smear.
Rectal Scraping
To obtain a sample by rectal scraping, the rectum is cleaned of feces, use of lubricant gel is minimized or avoided, and a rigid instrument such as a conjunctival scraper or chemistry spatula is used to obtain the specimen (Box 18-1 and Figure 18-3). The sample should be obtained cranially enough to reach the rectum, avoiding sampling of the anal mucosa; pressure should be applied firmly enough to sample the mucosa, rather than just the surface material, while being careful not to perforate the rectal wall. Slides are prepared, dried, and stained in a manner similar to the process for a fecal smear.
Esophagus
Normal Esophagus
The esophagus consists of a mucosal epithelium, submucosa, and a muscular wall. The esophageal mucosa is lined by nonkeratinizing stratified squamous epithelium. Submucosal mucous glands are present throughout the esophagus in the dog and are located only at the pharyngeal–esophageal junction in the cat.6
Cells from multiple layers of the squamous epithelium may be identified in cytologic specimens (Figure 18-4). Progressing from superficial to deep, superficial squamous cells are angular with a pyknotic to absent nucleus, intermediate cells are somewhat angular with a larger nucleus, and deep intermediate and parabasal cells have a smaller volume of more deeply basophilic cytoplasm with rounded borders.7 Cytology of normal esophageal brushings and washings consists of predominantly intermediate squamous cells with occasional superficial squamous cells. Cells from the deeper layers, including deep intermediate cells, parabasal cells, and rarely submucosal glandular epithelial cells, may also be seen cytologically, depending on the aggressiveness of the sampling technique, but this finding is typically an indicator of disease.8 Oropharyngeal contamination may be seen in normal or abnormal esophageal samples and may consist of a mixed bacterial population including Simonsiella, and, rarely, respiratory epithelial cells. Simonsiella is a Gram-negative large (6-8 micrometers [µm] long and 2-3 µm wide) rod-shaped bacteria that contains segmented groups of cells aligned face-to-face in juxtaposition, giving it a barcode-like or stacked-disk appearance.
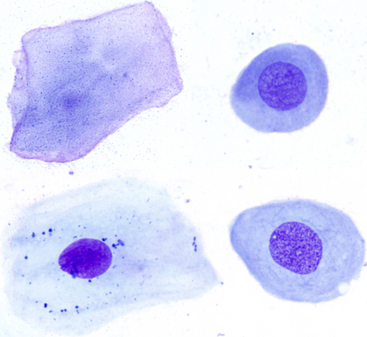
Figure 18-4 Esophageal brushings from normal dogs; various stages of esophageal squamous cells.
Beginning at the top right and moving clockwise: A parabasal cell, a deep intermediate cell, an intermediate cell and a superficial cell. Intermediate cells are most numerous in normal esophageal samples and, compared with superficial cells, are characterized by a less angular shape, a similar amount abundant cytoplasm that is nonkeratinized and a round, medium-sized nucleus. (Wright-Giemsa stain. Original magnification 50× objective.) (Slides courtesy of Sally Bissett.)
Esophageal Inflammation
Esophagitis occurs with injury to the esophageal mucosa due to a variety of underlying causes, including foreign bodies, infectious etiologies, and mucosal irritants (Box 18-2). Although esophagitis often has an erosive or ulcerative component, lack of a discernible superficial lesion at endoscopy does not rule out underlying esophagitis.
In addition to ingestion of substances damaging to the esophageal mucosa, an important cause of esophagitis with mucosal injury is reflux esophagitis. This condition, which is most common in the distal esophagus, is the effect of gastric acid, pepsin, and possibly bile salts and pancreatic enzymes on the esophageal mucosa.9 Reflux of these substances into the esophagus may occur with relaxation of the lower esophageal sphincter under anesthesia, a hiatal hernia, or chronic vomiting.10 In addition to esophageal inflammation, a possible sequela of gastroesophageal reflux is metaplasia of the distal esophageal stratified squamous epithelium to a more acid-friendly simple columnar epithelium with interspersed goblet cells (Figure 18-5). This lesion has been reported in dogs and cats; grossly or endoscopically, these may range from a region of hyperemia to a polypoid mass, which could be mistaken for neoplasia (Table 18-2).11 In humans, this condition is known as Barrett esophagus, and the lesion may progress and transform into a distal esophageal adenocarcinoma.
TABLE 18-2
Benign Non-Neoplastic Lesions That May Be Confused for Neoplasia
| Organ | Lesion |
| Esophagus | Barrett esophagus (metaplasia resulting from reflux esophagitis) |
| Parasitic granuloma (Spirocerca lupi) | |
| Stomach | Chronic hypertrophic gastropathy |
| Chronic hypertrophic pyloric gastropathy | |
| Pyloric stenosis | |
| Granulomatous gastritis (pythiosis) | |
| Idiopathic eosinophilic gastrointestinal masses | |
| Schirrous eosinophilic gastritis | |
| Feline gastrointestinal eosinophilic sclerosing fibroplasia | |
| Gastric polyps | |
| Intestine | Granulomatous enteritis or colitis (histoplasmosis, pythiosis, feline infectious peritonitis, protothecosis) |
| Histiocytic ulcerative colitis of Boxers | |
| Idiopathic eosinophilic gastrointestinal masses | |
| Feline gastrointestinal eosinophilic sclerosing fibroplasia | |
| Intestinal polyps |
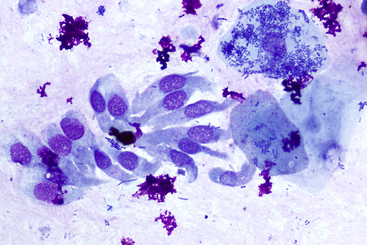
Figure 18-5 Canine brush cytology of a midesophogeal lesion; esophageal metaplasia.
The presence of uniform columnar epithelial cells (left side of image) indicates metaplasia has occurred. Note (in the right side of the image) the three superficial squamous epithelial cells with adhered bacteria and free bacteria in the background, indicating oropharyngeal contamination, and the small scattered clumps of magenta extracellular material consistent with lubricant gel. This pet was suffering from chronic vomiting. (Wright-Giemsa stain. Original magnification 50× objective.)
Cytologic findings with esophagitis are typically nonspecific with the presence of neutrophils amid the squamous epithelial cells. The presence of eosinophils in esophageal cytology may occur with neoplastic, parasitic, or fungal disease; with reflux esophagitis; as a part of eosinophilic gastroenteritis; or with eosinophilic esophagitis (Figure 18-6). Eosinophilic esophagitis has been reported in dogs, may be associated with allergic skin disease, and is a diagnosis of exclusion after eliminating the aforementioned causes of eosinophils within an esophageal sample.12
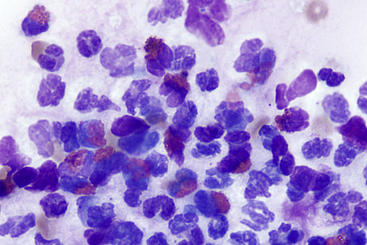
Figure 18-6 Esophageal brushing from a dog; eosinophilic esophagitis.
The sample has a mix of eosinophils and neutrophils on a background of mucus. Eosinophilic inflammation of the esophagus is not specific for the entity of eosinophilic esophagitis and other causes such as neoplasia, parasitic or fungal disease, reflux esophagitis, and eosinophilic gastroenteritis must be ruled out. In this case, no improvement was seen following treatment with a proton pump inhibitor, but significant improvement occurred following corticosteroid administration. (Wright Giemsa stain. Original magnification 100× objective.)
Esophageal Neoplasia
Primary esophageal neoplasia, which is rare in dogs and cats, includes squamous cell carcinoma and smooth muscle tumors, with rare reports of adenocarcinoma, neuroendocrine carcinoma, primary extraskeletal osteosarcoma, or plasma cell neoplasia.13,14 Fibrosarcoma and osteosarcoma associated with Spirocerca lupi infection is discussed above (see “Esophageal Inflammation”). Rarely, esophageal involvement of canine oral papillomavirus infection may occur.9
Squamous cell carcinoma arising from the esophageal mucosal epithelium most commonly occurs in the middle third of the esophagus and is typically an ulcerated plaque with circumferential esophageal thickening.13 Cytologically, this tumor resembles squamous cell carcinoma elsewhere in the body and often will have superimposed inflammation or evidence of superficial infection. Esophageal adenocarcinoma is rare but may arise from the submucosal esophageal glands or from regions of glandular metaplasia, as with reflux esophagitis. Leiomyomas in the esophagus are more common in dogs and are most commonly found in the outer muscular layer of the distal esophagus at the gastroesophageal junction.14
Stomach
Normal Stomach
The stomach in dogs and cats is glandular, and, from proximal to distal, contains cardiac, fundic, and pyloric regions and consists of the mucosa, muscularis mucosae, submucosa, and smooth muscle wall. The largest portion of the stomach is the fundus, which consists of a surface columnar foveolar epithelium with subjacent glandular cells, including parietal cells and chief cells, which secrete hydrochloric acid and pepsinogen, respectively (Figure 18-7). The pyloric region consists of a similar surface epithelium with predominantly mucous glands in the deeper mucosa (see Figure 18-7).
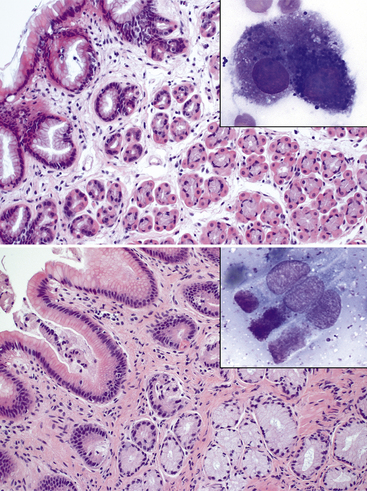
Figure 18-7 Stomach from a dog.
Top: The mucosa of the fundus is covered by a simple columnar epithelium with vacuolated cytoplasm (foveolar epithelium) that invaginates to form gastric pits. Deeper within the mucosa, glandular structures are present, with an inner rim of cuboidal basophilic to vacuolated chief cells (pepsinogen-secreting cells) and an outer rim of round eosinophilic parietal cells (hydrochloric acid-secreting cells). (H&E stain. Original magnification 20× objective.) The inset of a cytologic specimen has a chief cell with basophilic to purple cytoplasmic granules (right) and likely a parietal cell with pink cytoplasmic granules (left) (Modified Wright stain) Bottom: The mucosa of the pylorus is covered by a similar epithelium to the fundus, but the deeper mucosa contains predominantly mucus-secreting glands lined by pale, highly vacuolated epithelial cells. (H&E stain. Original magnification 20× objective.) The inset of a cytologic specimen has few mucus-secreting columnar epithelial cells, which contain apical pink-magenta cytoplasmic mucus-containing granules. (Wright-Giemsa stain. Original magnification 100× objective.)
Normal gastric cytology usually consists of small, to rarely large, sheets of surface epithelium that has a characteristic honeycomb appearance (Figure 18-8). These columnar cells have round to oval basally oriented nuclei with an abundant amount of finely vacuolated cytoplasm.7 Parietal and chief cells may be seen in gastric cytology samples collected from brushing techniques that access the deeper mucosal tissue (see Figure 18-7). Parietal cells have abundant granular eosinophilic to vacuolated cytoplasm, whereas chief cells have many well-staining basophilic cytoplasmic granules.7,8 Particularly in the pyloric region, mucus-secreting cells may be identified in cytologic samples (see Figure 18-7).
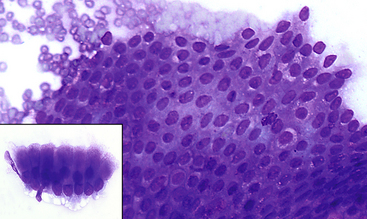
Figure 18-8 Normal gastric epithelium.
A large cluster of normal epithelium is present. Cells are uniform and have a honeycombed appearance within the cluster. The inset image is of a smaller cluster in which the columnar appearance of the cells can be appreciated. Cells are uniform with basilar oriented small round nuclei and abundant cytoplasm. (Modified Wright stain.)
Helicobacter spp. are spiral bacteria that are commonly identified in samples of the gastric surface of dogs and cats, often in close association with surface mucus (Figure 18-9). The possible clinical significance of finding Helicobacter organisms in the stomach is discussed below (see “Gastric Inflammation”). Gastric samples may be easily contaminated by food material, or material from the oral cavity and esophagus, which is indicated by the presence of Simonsiella organisms and squamous epithelial cells. Ciliated columnar respiratory epithelium may be noted if the patient swallowed sputum, whereas red blood cells (RBCs) may be identified with traumatic sample collection and blood contamination.
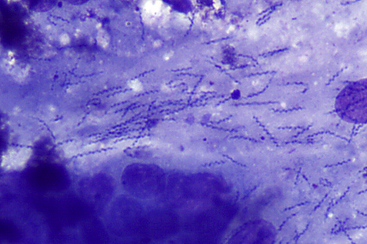
Figure 18-9 Pyloric region of the stomach of a dog; spiral bacteria consistent with Helicobacter spp.
The S-shaped spiral bacteria are embedded in thick streaming clumps of mucus; note the gastric epithelial cells at the bottom of the image. The significance of this finding is unknown, since Helicobacter spp. is commonly found in the stomachs of dogs and cats and may or may not be associated with inflammation. (Wright-Giemsa stain. Original magnification 50× objective.)
Gastric Inflammation
Gastritis is a nonspecific finding that may occur with a variety of causes (Box 18-3).15 The majority of gastritis cases in dogs and cats are likely a component of inflammatory bowel disease (IBD), which may have predominantly lymphoplasmacytic, eosinophilic or granulomatous inflammation, although the presence of inflammation is not specific for IBD. Normal endoscopic appearance of the gastric mucosa does not rule out underlying inflammation.
Neutrophilic Inflammation
Neutrophilic inflammation in gastric samples may occur with a variety of lesions but is seen most often with gastric ulcers; for a more complete list of common causes, see Table 18-3. Ulcers in the stomach or proximal small intestine may occur as a result of mechanical or chemical irritation, drug administration, or hormone secretion (i.e., gastrin hypersecretion or histamine release). Ulceration with nonsteroidal anti-inflammatory drug (NSAID) administration is secondary to compromise of mucosal protective mechanisms, whereas with excess exogenous or endogenous corticosteroids, mucosal perfusion is reduced.9 Uremic gastropathy is not typically an inflammatory lesion histologically but may cause gastric congestion, hemorrhage, and edema, possibly with ulceration, necrosis, and mineralization of the mucosa.9
TABLE 18-3
Causes of Gastric Inflammation
| Type of Inflammation | Disease Process | Potential Causes |
| Neutrophilic | Mechanical ulceration | Gastric foreign body, hairball |
| Chemical gastritis | Ingestion of irritating plants or chemicals | |
| Gastric or gastrointestinal (GI) ulcers | Hypersecretion of acid (liver disease, gastrin-secreting tumor) Histamine release (mast cell tumor, medications and hormones) Drug therapy (nonsteroidal anti-inflammatory drugs [NSAIDs], corticosteroids) Sepsis, burns, hypoadrenocorticism, surgery | |
| Lymphoplasmacytic | Inflammatory bowel disease | |
| Hyperplastic or hypertrophic conditions | Chronic hypertrophic gastropathy of Drentsche Patrijshond and Basenji dogs Chronic hypertrophic pyloric gastropathy Pyloric stenosis Benign gastric polyps | |
| Atrophic conditions | Chronic atrophic gastritis of Norwegian Lundehund dogs | |
| Healing gastric ulcers | ||
| Helicobacter infection | ||
| Parasite infestation | Physaloptera and Gnathostoma in dogs and cats Ollulanus and Cylicospirura in cats | |
| Secondary to other conditions | Neoplasia | |
| Eosinophilic | Inflammatory bowel disease | |
| Allergy or hypersensitivity disorders | ||
| Neoplasia | Mast cell tumor, T-cell lymphoma | |
| Infectious agents | Pythiosis, Toxocara canis larval migration | |
| Miscellaneous | Feline hypereosinophilic syndrome Canine idiopathic eosinophilic gastrointestinal masses Scirrhous eosinophilic gastritis Feline gastrointestinal eosinophilic sclerosing fibroplasia | |
| Granulomatous | Inflammatory bowel disease | |
| Infectious etiologies | Mycobacteriosis, histoplasmosis, pythiosis |
Lymphoplasmacytic Inflammation
Lymphoplasmacytic gastritis is the most common histopathologic finding in abnormal gastric biopsies in dogs and has a variety of causes and disease associations, including IBD, hyperplastic, hypertrophic, or atrophic conditions, and others (Figure 18-10).16 For a more complete list of potential causes, see Table 18-3.
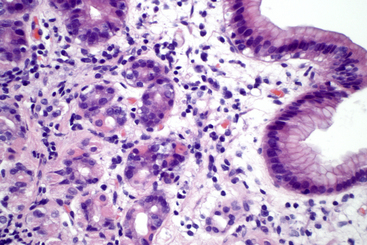
Figure 18-10 Stomach from a dog; lymphoplasmacytic gastritis as a component of inflammatory bowel disease.
Within the lamina propria of the superficial gastric mucosa, increased numbers of lymphocytes and plasma cells are seen, with mild edema and increased connective tissue. (H&E stain. Original magnification 40× objective.)
Several hyperplastic or hypertrophic gastric conditions occur in dogs (see Table 18-3). In addition to a lymphoplasmacytic inflammatory component, these conditions often have a component of mucosal epithelial proliferation, with or without thickening or muscular hypertrophy of the stomach wall; therefore, increased mucosal epithelium may be noted cytologically in these conditions. As a result of their gross or endoscopic appearance as a diffuse or regional masslike thickening of portions of the stomach, these hyperplastic and hypertrophic lesions may be confused with gastric adenocarcinoma (see Table 18-2).13 For this reason, it is important to consider these benign conditions when increased mucosal epithelium is seen in cytology specimens.
Helicobacter spp. are highly prevalent spiral bacteria found in the stomach of dogs and cats; however, it is controversial whether a causal association exists between the presence of Helicobacter and the presence of gastritis in these species (see Figure 18-9 and Table 18-1). In cats, an association seems to exist between Helicobacter and lymphoid follicle formation or epithelial proliferation in the stomach, and possible associations have been made between Helicobacter infection and gastric mucosal-associated lymphoid tissue (MALT) lymphoma.17,18 Helicobacter may be present in higher numbers and easier to identify in cytologic specimens than in histologic samples because of their presence in the surface mucus, which is readily sampled for cytologic evaluation.
Eosinophilic Inflammation
Eosinophilic inflammation in stomach samples may occur as a component of IBD or with other diseases typically associated with eosinophils (see Table 18-3). Other conditions with a predominance of eosinophils include idiopathic eosinophilic GI masses, scirrhous eosinophilic gastritis, and feline GI eosinophilic sclerosing fibroplasia; an important feature of these diseases is their tendency to form a thickened or masslike region in the stomach, giving the impression of neoplasia (see Table 18-2). The term idiopathic eosinophilic gastrointestinal masses (IEGM) refers to a condition in dogs, with a predisposition in Rottweiler dogs, in which one or multiple mass lesions consisting of eosinophilic inflammation are present in the GI tract, with intervening eosinophil-free regions.19 Scirrhous eosinophilic gastritis in dogs is a thickening of the gastric wall with granulation tissue and eosinophils.13 Feline gastrointestinal eosinophilic sclerosing fibroplasia is a masslike lesion, most commonly at the pyloric sphincter but also common at the ileocecocolic junction, and it may also involve the mesenteric lymph nodes. Cytologically, this lesion has either increased eosinophils, alone or in combination with large spindle cells amid pink, extracellular matrix, with neutrophils and intracellular and extracellular rod-shaped or coccoid bacteria with fewer lymphocytes, plasma cells, and mast cells (Figure 18-11).20
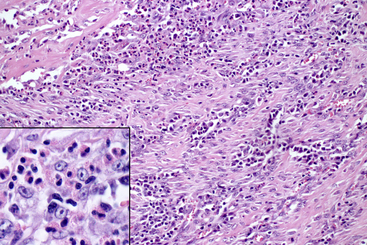
Figure 18-11 Pyloric region of the stomach from a cat; feline gastrointestinal eosinophilic sclerosing fibroplasia.
The majority of the masslike lesion is composed of thick anastomosing bands of dense collagenous or fibrous stroma, with plump proliferating fibroblasts and intervening aggregates of numerous eosinophils. The inset is a closer view of the inflammatory cells and fibroblasts with predominantly eosinophils and fewer macrophages, lymphocytes, and plasma cells. (H&E stain. Original magnification 20× objective. Inset: H&E stain. Original magnification 100× objective.) (Case from Joint Pathology Center Veterinary Pathology Services: Wednesday Slide Conference 2011-12, Conference 15 Case 1.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree