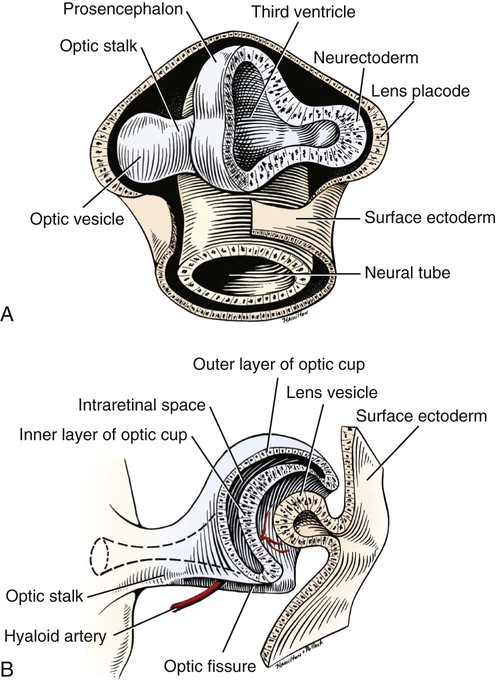
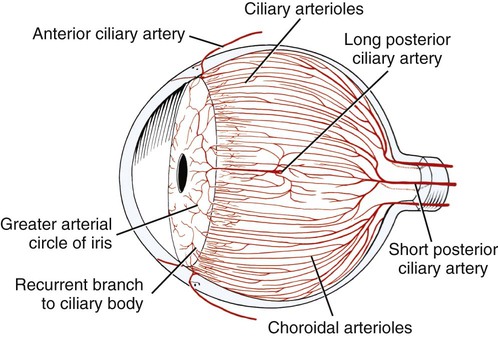
Christopher J. Murphy, Donald A. Samuelson and Roy V.H. Pollock The eye (organum visus) (Fig. 21-1) develops as a neuroectodermal outgrowth of the embryonic prosencephalon that contacts surface ectoderm and is enveloped by induced mesodermal and neural crest mesenchyme. The definitive eye and its adnexa are contained within an orbit that is only partly bony. Associated with the bulb of the eye are extraocular muscles that move it; periorbital fascia and fat that surround and cushion it; eyelids and conjunctivae that protect it; and a lacrimal apparatus that keeps its surface moist, provides the first barrier to infection, and helps to nourish the cornea. There is considerable variation between breeds in regard to the position of the eyes, the size of the orbit, and the size and shape of the palpebral opening (Fig. 21-23). Aguirre et al. (1972) studied the early development of the dog’s eye and its adnexa from day 15 to functional maturity using serially sectioned embryos in the Cornell University Collection (Evans & Sack, 1973). Subsequent studies involved histologic examination of fetuses removed from the uterus 25, 28, 30, 33, and 35 days post coitum (Boevé et al., 1988; Boevé et al., 1989). The first indication of the formation of the eye is seen as an optic sulcus on the neural fold rostral to the notochord on each side. The neuroectoderm surrounding this sulcus proliferates rostrolaterally to form the optic vesicle, a hollow diverticulum of the prosencephalon. As the optic vesicle forms, its caudal surface is contacted by mesodermal mesenchyme and its peripheral surface is surrounded by superficial ectoderm. Shortly thereafter, migrating neural crest cells contribute to the forming vesicle and future orbital tissues. The anterior portion of the vesicle invaginates to form an optic cup with the concomitant formation of the lens placode in the adjacent surface ectoderm. The optic cup assumes a rounded appearance by day 30. There is an optic fissure along the ventral meridian where the lateral and medial folds of the optic cup meet and eventually fuse (Fig. 21-2B). The occurrence of the fissure allows for the penetration of the vascular mesoderm. Failure of the fissure to close results in defects of one or more of the tunics of the eye (colobomata). Such defects are common in the Collie breed as part of the heritable Collie eye syndrome. The connection of the optic cup to the brainstem lengthens and attenuates as growth proceeds, forming the optic stalk, which will later become the optic nerve. Anteriorly, the optic vesicle induces the overlying surface ectoderm to proliferate, forming the lens placode, which is present by gestational day 15. As the optic vesicle invaginates, the placode also invaginates into the optic cup. By day 25, it pinches off from the surface ectoderm to form the lens vesicle, the anlage of the crystalline lens (see Fig. 21-2B). The first anlage of the lens capsule is evident by day 25 (Boevé et al., 1988). The remaining surface ectoderm becomes the epithelium of the cornea. The deeper stromal and posterior epithelial layers of the cornea are derived from neural crest mesenchymal cells. The hyaloid artery is present at day 25, arising from the mesenchyme surrounding the optic cup. It enters the posterior end of the optic fissure to supply the inner surface of the cup and the mesenchyme filling the optic cup becomes the primary vitreous (see Fig. 21-2B). The hyaloid artery grows anteriorly and reaches the posterior lens surface by day 28, where it branches extensively to form the posterior portion of the tunica vasculosa lentis, a plexus of vessels derived from the anterior ciliary vessels that completely surrounds the lens by day 30. Secondary vitreous, secreted by the glial component consisting of Müller cells within the inner layer of the optic cup, surrounds the primary vitreous beginning at approximately gestational day 26. With the growth of the eyeball, the secondary vitreous continues to elaborate and the primary vitreous becomes reduced to a narrow funnel (Cloquet canal) between the optic nerve and posterior lens surface (Fig. 21-1). The hyaloid vessels between the optic disc and lens begin to atrophy at approximately day 45 with remnants commonly present until 10 or 11 days after birth. The retinal arteries of the adult are derived from the portion of the hyaloid vasculature that supplied the inner layer of the optic cup. The cavity of the optic vesicle, originally continuous with the third ventricle of the brain, is obliterated on approximately day 33, when the inner and outer walls of the optic cup fuse. The outer layer becomes the retinal pigment epithelium. The inner layer proliferates to form all layers of the neurosensory retina. Axons from the ganglion cells of the innermost layer grow toward the optic stalk, which they invade on approximately gestational day 30, and follow toward the brainstem. Maturation of the retina proceeds from central to peripheral and is not complete until 8 weeks after birth (Gum et al., 1984; Shively et al., 1971). The epithelia of the ciliary body and the posterior surface of the iris are also derived from the neuroectoderm of the inner layer of the optic cup, but are nonvisual (pars ceca retinae). The richly vascular mesenchyme surrounding the anterior tunica vasculosa lentis forms the stroma of the iris. The central area of the iris (pupillary membrane) is thin and normally completely atrophies by 14 days after birth, forming the pupil. Incomplete atrophy, resulting in persistent pupillary membranes, has been observed as a heritable defect in Basenji dogs (Roberts & Bistner, 1968). The dilator and sphincter muscles of the iris differentiate from the neuroectodermal pars iridica retinae. Rarefaction of the mesenchyme between that which forms the stroma of the iris and that which forms the substantia propria and posterior epithelium of the cornea is evident by day 45. Progressive rarefaction forms a cavity that fills with aqueous humor to become the anterior chamber of the adult eye (Fig. 21-3). External to the vascular tunic, the mesenchyme (primarily of neural crest origin) condenses to form the fibrous sclera, which is homologous to the dura mater of the brain. Neural crest cells are the source of the posterior corneal epithelial cells of the cornea as well as stromal fibroblasts (keratocytes). The extraocular muscles are derived from somitomere mesoderm caudal to the developing eye vesicle. Although the myofibers of these muscles are mesodermal, the connective tissues associated with them are of neural crest origin. Gilbert (1947) demonstrated that the extrinsic ocular muscles of the cat arise from three distinct but closely approximated anlagen that are homologous with the premandibular, mandibular, and hyoid head cavities of lower vertebrates. The same is probably true for the dog. The eyelids appear as folds superior and inferior to the eye on approximately day 25 of gestation. These enlarge, grow over the cornea, and narrow the palpebral fissure by approximately half on day 35. The eyelids completely cover the cornea and fuse by day 40 (Evans, 1974). The superficial musculature of the lids, the m. orbicularis oculi, forms from the platysma sheet and is recognizable by approximately day 40. Huber (1922) has described the postnatal development of the platysma derivatives. Dogs are born with the lid margins still adherent to one another. Final maturation of the eye occurs after birth; most notable are changes in the retina, iridocorneal angle, corneal epithelium, and tapetum. The fused lids normally separate approximately 2 weeks postpartum, and the palpebral fissure is able to be opened. Premature separation of the lids results in severe ophthalmitis, apparently as a result of the immaturity of the lacrimal apparatus (Aguirre & Rubin, 1970). The retina of the dog, as in many precocial species, is not fully developed at birth. Differentiation of the multicellular inner layer of the optic cup into inner (marginal) and outer (nucleated) layers to form the precursor of the sensory retina does not occur until embryonic days 25-28. Intracellular pigment granules may be observed at the periphery of the outer layer near the ora serrata, which will later form the pigment epithelium. By day 30, the anterior part of the pigment epithelium consists of cells arranged in pseudostratified columnar fashion and the nerve fiber layer may be observed at the posterior pole of the retina. The inner and outer neuroblastic layers of the retina may be distinguished posterior to the equator by day 30. In the posterior region, the nerve fiber layer becomes prominent and the axons join to form the optic nerve. By day 35, the retinal pigment epithelium becomes pigmented posterior to the equator of the optic cup. The photoreceptors are not developed until approximately 16 to 35 days after birth (Aguirre et al., 1972; Miller et al., 1989; Perry, 1940; Shively et al., 1971; Whitely and Young, 1985). This lag in photoreceptor maturation is reflected by the electrical activity of the retina in response to photic stimulation (Gum et al., 1984). Vascularization of the retina occurs postnatally (Flower et al., 1985). The eyeball (bulbus oculi) is formed by three concentric coats: the fibrous tunic (tunica fibrosa bulbi), the middle vascular tunic (tunica vasculosa bulbi), and the inner nervous tunic (tunica interna bulbi). In the dog, the eyeball is nearly spherical, differing little in its sagittal, transverse, and vertical diameters. The size of the eyeball varies among breeds, but the diameter is usually approximately 20 to 22 mm (Table 21-1). In one study, the radius of the canine eye varied across breeds from 9.56 to 11.57 mm and was correlated with the width and length of the skull (McGreevy et al., 2004). TABLE 21-1 Ocular Dimensions of the Dog Eye* SD, Standard deviation. *These values are taken from the literature and are based on measurements made in vivo or from freshly excised tissues. A summary of the older literature pertaining to ocular dimensions, commonly made from fixed specimens, is available in Bayer (1914). †1, Gaiddon et al., 1991; 2, Schiffer et al., 1982; 3, Gwin et al., 1982; 4, Murphy et al., 1992 (from adult German Shepherd Dogs); 5, Mutti et al., 1999 (from adult Labrador Retrievers); 6, Kafarnik et al., 2007; 7, Gilger et al., 2005 (average of multiple modes of measurement); 8, Kreuzer and Sivak, 1985. The transparent cornea forms the anterior one-fourth of the eyeball, and because it has a smaller radius of curvature (approximately 8.5 to 9 mm) than the rest of the eye, it bulges anteriorly (see Fig. 21-3). The vertex of the cornea is designated the anterior pole of the eye (polus anterior). The point directly opposite this is the posterior pole (polus posterior). The latter is a geometric point and does not correspond to the exit point of the optic nerve, which lies ventrolateral to the posterior pole. The line connecting the anterior and posterior poles and passing through the center of the lens is the axis bulbi (Fig. 21-3). In the mesaticephalic dog the axis forms an angle of approximately 30 degrees with the median plane. The angle is greater in the brachycephalic breeds (see Fig. 21-17). Lines connecting the anterior and posterior poles of the eye on the surface of the globe are designated meridians. The equator of the globe is its maximum circumference located midway between the poles (Fig. 21-3). Because the eye is essentially spherical, the common anatomic terms of direction are not applicable for certain structures, such as the retinal layers. In such cases, the terms inner and outer are used with reference to the center of the bulb. The sclera consists of a dense network of collagen and elastic fibers and their attendant fibrocytes. It varies in thickness, being greatest in the region just posterior to the corneoscleral junction, where it receives the insertions of the rectus and oblique muscles and contains the scleral venous plexus. The ciliary muscle is attached to a small ridge of fibrous tissue that forms a ring (anulus sclerae) on the inner surface of the sclera posterior to the iridocorneal angle (Fig. 21-9). The mean scleral surface area of canine eyes is reported to be 12.87 (±2.24) cm2 (Gilger et al., 2005).The thickness of the sclera is only 0.34 (±0.13) mm near the equator (Gilger et al., 2005). It becomes thicker again on the posterior aspect of the globe. Where the optic nerve leaves the eyeball, the sclera is sievelike (area cribrosa sclerae). Here the collagen, elastic, and reticular fiber bundles of the sclera form a net through the interstices of which the optic nerve myelinated axons pass. The trabeculae of the area cribrosa continue caudally as the prominent connective tissue septae of the optic nerve. The dura mater surrounding the optic nerve (vagina externa n. optici) is continuous with the outer layers of the sclera at the periphery of the area cribrosa, and with the periorbita and dura mater encephali at the optic canal. The cornea forms the anterior segment of the fibrous tunic. The normally transparent cornea contains 80% (±2%) water (Scott & Bosworth, 1990). The mean full thickness of the adult canine cornea is approximately 600 µm. In one study, central corneal thickness was reported to be 585 (±79) µm. In dogs less than 1 year of age it was 555 (±64) µm, whereas in older dogs it was 606 (±85) µm (Kafarnik et al., 2007) (see Table 21-1). Its thickness increases with age (Gwin et al., 1982). In another study the mean central corneal thickness of 43 dogs was found to be 611 µm (Lynch & Brinkis, 2006). Measurements made with optical and ultrasonic pachymeters in vivo show the cornea to be uniformly thinner axially than at the limbus (see Fig. 21-3 and Table 21-1). The radius of curvature of the living dog cornea is approximately 8.5 to 9.0 mm (Gaiddon et al., 1991; Murphy et al., 1992). Large-breed dogs have been shown to have a slightly flatter cornea (a larger radius of curvature) than small or medium-sized breeds (Gaiddon et al., 1991). The cornea of the dog is very slightly oval; the mediolateral dimension is usually approximately 10% greater than the dorsoventral dimension, which is typically 16 to 18 mm in an average sized dog. Classically, the cornea is described as consisting of five layers: the anterior epithelium, the anterior limiting lamina (Bowman layer), the substantia propria, the posterior limiting lamina (Descemet membrane), and the posterior epithelium (endothelium). The most variable of these layers among vertebrates is the presence of an anterior limiting lamina. Shively and Epling (1970), in a study of the fine structure of the canine cornea, were unable to demonstrate a distinct layer comparable to the anterior limiting lamina (Bowman layer). This was confirmed by Morrin et al. (1982), who described the ultrastructure of the Beagle cornea. They did describe, however, the subepithelial stroma (for a thickness of approximately 9 µm) as being hypocellular and to contain randomly oriented collagen bundles. The anterior epithelium of the cornea is approximately 19 cells thick and consists of three distinct layers. A single layer of columnar basal cells attaches to the underlying stroma through a specialization of the extracellular matrix, the anterior corneal basement membrane. The anterior corneal basement membrane of the dog has been documented to possess a rich three-dimensional “felt-like” topographic architecture composed of nanoscale to submicron intertwining fibers, pores, and elevations (Abrams et al., 2002). The surface topographic features as well as the intrinsic compliance (relative stiffness) of basement membranes have been shown to profoundly modulate a wide menu of corneal epithelial cell behaviors (Abrams et al., 2002; Last et al., 2009). Over the basal cell layer are approximately three layers of polygonal wing cells. The superficial layers are composed of flattened squamous cells. The most superficial layer of squamous cells is in direct contact with the precorneal tear film and has a microplicated surface. The rugae of this surface are thought to be important in anchoring the precorneal tear film and may also play a role in transport processes by amplification of the membrane surface. Both the anterior and posterior corneal epithelia, but especially the latter, regulate the degree of the hydration of the substantia propria by an active transport mechanism. Disruption of either epithelium results in corneal edema with more severe edema developing with involvement of the posterior epithelium. The canine corneal epithelium contains carbonic anhydrase, which may facilitate the elimination of metabolic carbon dioxide against small concentration gradients via catalytic conversion to bicarbonate (HCO3–) (Conroy et al., 1992). The opioid growth factor peptide [met5]enkephalin and its corresponding receptor are found in the canine cornea where they may play a role in homeostasis and repair of the corneal epithelium (Robertson & Andrew, 2003). Toll-like receptor 4, which is important for recognizing highly conserved molecular patterns of pathogens, is expressed in the canine cornea (Wassef et al., 2004). The nucleotide-binding oligomerization domain (NOD) proteins NOD1 and NOD2 are also expressed in the canine corneal anterior and posterior epithelia where they serve as signaling receptors of the innate immune system (Scurrell et al., 2009). NOD1 and NOD2 are also found in the conjunctival and nonpigmented iridal epithelium. Cholestyramine, a highly nonpolar dehydration product of cholesterol is present in normal dog corneas where it accounts for 20% to 25% of the total steroid-sterol present (Cenedella et al., 1992). The transparency of the cornea remains incompletely understood. There are three elements thought to participate in achieving and maintaining corneal transparency: (1) uniform sizing and spatial distribution of the collagen fibrils that compose the extracellular matrix of the stroma, (2) the distribution of media of differing refractive indices within the stroma, and (3) the expression of crystallins by the stromal cells of the cornea (Jester, 2008). Other factors that contribute to corneal transparency are the lack of pigment, vessels, and large myelinated nerve fibers. Any disruption of the highly ordered structure of the collagen fibers, such as by edema or by scar tissue, results in a loss of transparency. The cornea, normally avascular, is nourished by the capillary loops at the corneoscleral junction (limbus), the precorneal tear film, and the aqueous humor. Recently it has been suggested that the avascularity of the cornea is due to the expression of soluble vascular endothelial growth factor (VEGF) receptor-1 within corneal tissues. This receptor serves as a “trap,” binding available VEGF, preventing it from inciting vessel formation (Ambati et al., 2006). The substantia propria is composed of highly ordered collagen fibrils with interspersed cellular elements. These fibrils are of uniform small diameter and are arranged in distinct lamellae. The cornea is easily dissected along these lamellar planes. The majority of fibrils within a lamella run parallel to each other and to the corneal surface, although the fibrils in each lamella run at an angle to those in the other layers. Additionally, although not verified to date in the dog cornea, recent work in the human cornea using second harmonic imaging demonstrates a high degree of lamellar interweaving especially in the anterior stroma (Morishige et al., 2007). The fibrocytes of the cornea (keratocytes) are flattened between the lamellae. The posterior limiting lamina (Descemet membrane) is the exaggerated basement membrane of the posterior epithelium of the cornea. It is approximately two times thicker in the dog than in humans (Engerman & Colquhoun, 1982). The posterior epithelium of the cornea is composed of a simple cuboidal epithelium, individual cells of which are mostly hexagonal in outline with a mean cell area of 395 (±36) µm2 (Pigatto et al., 2006). The mean endothelial cell density of the canine cornea has been reported as 3175 (±776)/mm2 (Kafarnik et al., 2007) and 2555 (±240) cells/mm2 (Pigatto et al., 2006). In dogs less than 1 year of age it has been reported to be 3641 (±752)/mm2, whereas in older dogs it is 2851 (±634)/mm2 (Kafarnik et al., 2007). The density of these cells has been shown to decrease with age and following intraocular surgery (Gwin et al., 1982, 1983; Yee et al., 1987). The morphologic characteristics of these cells have also been shown to be altered in some disease states such as diabetes (Yee et al., 1985). The ability of this layer to regenerate is species variable. Severe trauma to this layer of cells frequently results in chronic corneal edema. The cornea is innervated by branches of the ciliary nerves that arise from the ophthalmic nerve, a branch of the trigeminal nerve. Nerve branches to the cornea enter the anterior layers of the stroma at the limbus and soon lose their myelin sheaths as they converge toward the vertex. The corneal nerve plexus is formed by thick-, medium-, and thin-diameter nerve bundles. The mean nerve bundle length in 1 mm2 of cornea is 10.32 (±0.11) mm in adult dogs, which is significantly higher than 9.42 (±0.02) mm and 7.75 (±0.14) mm in young and old dogs, respectively (Lasys et al., 2003). In mesocephalic dogs, the mean density of subepithelial and subbasal nerve fibers is 12.39 (±5.25) mm/mm2 and 14.87 (±3.08) mm/mm2, respectively. These values are significantly higher than the corresponding values in brachycephalic dogs of 10.34 (±4.71) mm/mm2 and 11.80 (±3.73) mm/mm2, respectively (Kafarnik et al., 2008). The limbal plexus is a 0.8-1 mm network of superficial nerves around the peripheral cornea (Marfurt et al., 2001). The numerous origins of the limbal fibers include collateral branches of stromal and subconjunctival fibers in passage to the cornea, recurrent collaterals from the peripheral corneal plexus, and perivascular fibers associated with the rich limbal vasculature. The limbal plexus is morphologically subdivided into outer periscleral and inner pericorneal zones. The periscleral zone contains largely perivascular nerve fascicles and a stromal plexus with axons extending randomly through the limbal stroma. The pericorneal zone is a much denser meshwork of highly branched and anastomotic axons and small-diameter fascicles. Many inner zone fibers are intimately associated with vascular elements of the superficial limbal arcade, and others travel through the corneoscleral transition zone and anastomose with axons in the peripheral anterior stromal plexus. The limbal and conjunctival epithelia contain modest numbers of short, wavy, beaded, and mostly radially oriented axons. Most nerve fibers enter the peripheral cornea at the corneoscleral limbus in a series of 14-18 prominent, radially directed, superficial stromal nerve bundles containing 30-40 axons (Marfurt et al., 2001). These are located at regular intervals around the limbal circumference. Smaller nerve fascicles enter the peripheral cornea between and slightly superficial to the main bundles. Subsequently, the main stromal bundles undergo extensive dichotomous branching to form elaborate axonal trees. The distal branches of these trees are extensively joined at angular junctions to form a dense, anatomically complex stromal plexus that extends uninterrupted to all areas of the cornea. The latter plexus occupies approximately the anterior 0.4-0.5 mm half of the corneal stroma and can be further subdivided into posterior and anterior levels. The posterior level contains modest numbers of primarily small- to medium-diameter bundles and scattered individual axons. The anterior level is much more densely innervated and morphologically complex. A very fine meshwork of thin, preterminal axons occupies the region immediately beneath the epithelial basement membrane. In contrast to the highly innervated anterior stroma, the posterior corneal stroma of the dog is largely noninnervated. After entering the basal epithelial cell layer, most intraepithelial axons form preterminal arborizations known as epithelial leashes (Marfurt et al., 2001). Each epithelial leash comprises 2-6 axons attached to a single subepithelial fiber. Individual axons course horizontally to the surface and roughly parallel to each other through the basal epithelial cell layer tangential to the corneal for 1-1.4 mm. Most axons are less than 2.5 µm in diameter but range from 1.2-3.5 µm. As they travel horizontally through the basal epithelium they give rise to an abundance of thin, ascending branches that divide extensively to form irregular clusters of short terminal branches ending throughout the basal, wing, and squamous epithelial layers. Most axonal endings consist of single large bulbous terminal expansions. Trigeminal nerve innervation of the cornea is essential to maintaining homeostasis. Loss of sensory innervation apparently disrupts a trophic influence normally supplied by the ciliary nerves. Corneal denervation results in corneal ulceration, edema, and loss of stromal tissue (neurotrophic keratitis), even though eyelid function is unimpaired (Scott & Bistner, 1973). There is some evidence to suggest that the neuropeptide, substance P, associated with the corneal terminations of the trigeminal nerve, is the neuronal factor necessary to the maintenance of normal corneal health (Marfurt et al., 2001; Murphy et al., 1990; Murphy et al., 2001). The cornea also receives sympathetic innervation, and the anterior corneal epithelium of the dog, apparently independent of a neuronal source, is rich in acetylcholine (Gwin et al., 1979). An extensive review of corneal innervation including the dog is provided by Muller et al. (2003). At the corneoscleral junction, the anterior corneal epithelium is continuous with the bulbar conjunctiva (see later section on conjunctiva). The collagen fibers of the substantia propria become abruptly less ordered as they approach the sclera, with a resulting loss of transparency. The corneoscleral junction is oblique; the posterior aspect is more peripheral than the anterior (Fig. 21-4B). Immediately peripheral to the limbus, the posterior corneal epithelium reflects onto the anterior face of the iris, forming the iridocorneal (filtration) angle. The iridocorneal angle is a regional term that includes the most anterior internal aspect of the sclera, the most posterior internal aspect of the cornea, the most anterior external aspect of the ciliary body, the root of the iris, and all intervening tissue associated with these structures. In the embryo the iridocorneal angle is a smooth, unfenestrated fornix. Late in gestation and continuing in the early postnatal period, the tissue in this area undergoes progressive rarefaction until a long cleft extends the anterior chamber posteriorly between the base of the iris and the sclera (Aguirre et al., 1972; Martin, 1975; Samuelson & Gelatt, 1984a, b). Examination of 23 normal dogs (46 eyes) that were 5 (±2.73) years of age revealed a mean iridocorneal angle of 12.6 (±5.3) degrees (range, 5-29) and mean angle opening distance of 273.4 (±88.9) µm (range, 107-557) (Rose et al., 2008). This cleft is bridged by a network of fine collagenous pillars that in aggregate form the pectinate ligament (lig. pectinatum anguli iridocornealis). The heavily pigmented strands of the canine pectinate ligament exhibit large variations in size and thickness. They exist primarily as single strands but may fuse with adjacent strands or ramify into a characteristic branching pattern prior to inserting obliquely onto the corneal limbus (Simones et al., 1996). Because the strands are relatively slender and few in number, there are wide intertrabecular spaces. The iridocorneal angle is further divided into corneoscleral trabeculae and deeper uveal trabeculae. The region of the iridocorneal angle contains Schwalbe line cells, which possess secretory and epithelial characteristics that are associated with the nonfiltering portion of the corneoscleral trabecular meshwork and are considered to be a subpopulation of trabecular cells (Samuelson et al., 2001). The number of Schwalbe line cells declines gradually with age in normal dogs but more rapidly in dogs with glaucoma. The trabecular cells can actively phagocytose small particulate matter (Samuelson et al., 1984). Samuelson and Gelatt (1984a, b) and Bedford and Grierson (1986) have provided detailed anatomic descriptions of the aqueous outflow pathway in the dog. In many cases, the various reports concerning this region have conflicting findings and the terminology lacks uniformity (Samuelson, 1996). Bedford (1977) has described the clinical appearance of the iridocorneal angle using a special goniolens. The aqueous humor leaves the eye by filtering through the spaces between the corneoscleral trabeculae to aqueous collector vessels, the angular aqueous plexus, that join the scleral venous plexus, which, in turn, is drained by the anterior ciliary and vorticose veins (Samuelson & Gelatt, 1984b; Van Buskirk, 1979). The outermost corneoscleral trabeculae appear to contribute to the canine aqueous outflow barrier by compartmentalizing the glycosaminoglycans in the intervening spaces between trabeculae (Gum et al., 1993). They also prevent widening of the angle and hold the initial filtration structures in a relatively compressed state (Morrison & Van Buskirk, 1982). Dilation of the pupil (mydriasis) impedes the outflow of aqueous humor; the iridocorneal angle is narrowed by the increased thickness of the peripheral iris. Constriction of the pupil (miosis) opens the spaces of the iridocorneal angle and facilitates drainage (Mark, 2003). In certain breeds, notably the Basset Hound, the corneoscleral angle is dysplastic. The pectinate ligament is sheetlike with few openings (Martin, 1975), which impedes the outflow of the aqueous humor. Animals with this condition are thought to be predisposed to developing glaucoma. A positive relationship between pectinate ligament dysplasia and narrowing of the iridocorneal angle with the development of glaucoma has been demonstrated in English Springer Spaniels (Bjerkås et al., 2002). The vascular tunic (tunica vasculosa bulbi) is the thick middle coat of the eye, interposed between the retina and the sclera. It is commonly referred to as the uvea or uveal tract. The vascular tunic includes three contiguous parts, which, from posterior to anterior, are the choroid, the ciliary body, and the iris (Fig. 21-1). Its functions are numerous and include regulating the amount of light entering the eye through the pupil; producing the aqueous humor, which maintains the intraocular pressure and bathes the structures of the anterior segment; suspending the lens via zonula fibers; changing the visual focus via the ciliary body muscle; limiting the amount of scattered light within the eye (inner pigmented portion of uvea); increasing the photic stimulation of the retina under low light levels (tapetum lucidum of the choroid); and providing nutrition to structures within the eye (ciliary body and choroid). The tapetum is a specialized reflective layer of the choroid (Fig. 21-1). It is thought to increase the ability of the retina to function under low light levels. In addition to the reflection of incident light back through the overlying photoreceptor layer, it is also thought to increase photoreceptor stimulation by intrinsic fluorescence of its structure when stimulated by incident light (Bellhorn, 1990; Elliott & Futterman, 1963). A tapetum is present in all domestic mammals except the pig. In almost all mammals it is located within the middle-sized vessel layer of the choroid, interposed between the choriocapillaris and the large-sized vessel layer. It is cellular (tapetum lucidum cellulosum) in all carnivores, whereas it is collagenous (tapetum lucidum fibrosum) in all herbivores (Ollivier et al., 2004). It occupies approximately one third of the superior area of the choroid. Central areas of the canine tapetum have been reported to contain 9 to 11 layers of tapetal cells (Wen et al., 1985), or 15 to 20 layers (Lesiuk & Braekvelt, 1983). Cell numbers diminish to a single layer peripherally and next to the optic nerve. The cells are layered in a step-wise manner that form a brick-wall appearance. The zinc- and cysteine-rich tapetal cells are packed with highly refractive, membrane-bound tapetal rodlets. These rodlets are oriented parallel to the retina and are thought to be responsible for the tapetum’s reflectivity (Hebel, 1969, 1971). The unique structure of the tapetum makes it exquisitely sensitive to the toxic effects of a beta adrenergic blocking agent that may have no toxic effects whatsoever in Beagles that inherit an aplasia of the tapetum (Massa et al., 1984; Schiavo et al., 1984). Penetrating vessels are oriented radially and connect the choriocapillaris with the stromal vessels. The radial orientation of these penetrating vessels minimizes their potential interference on tapetal function. In dogs, the tapetum is in roughly the shape of a rounded right triangle with the hypotenuse resting on a dorsal plane and the right angle situated dorsally (Fig. 21-11). The medial angle is more acute than the lateral. In large breeds of dogs, the hypotenuse (inferior border) is usually inferior to the optic disc. In small breeds of dogs, the tapetum is relatively smaller and does not extend inferiorly to include the optic disc. In some toy breeds, the tapetum may be greatly diminished in area or may be entirely absent as a normal variation. A heritable lack of the tapetum has been described in the Beagle, in which tapetal cells are initially present but fail to develop normal tapetal rodlets (Bellhorn et al., 1975; Burns et al., 1988a; Burns et al., 1988b). The tapetum develops after birth. As the dog matures, the color of the tapetum changes from a slate gray to violet to red-orange at approximately 4 months of age (Rubin, 1974). The color is generally uniform except at the junction of the tapetal and nontapetal choroid, which may be quite irregular and demonstrate considerable pleochromism. The distinctive coloration of the tapetum is due to the optical phenomenon of thin film interference rather than the presence of specific pigments. In other words, tapetal coloration is structural rather than pigmentary. The vascular layer of the choroid is a plexus of arteries, arterioles, veins, and venules supported by a collagenous and elastic stroma and traditionally subdivided into an outer large-sized vessel layer and an inner middle-sized vessel layer. The outer large-sized vessel layer is the terminal branches of the ciliary arteries and the vorticose veins. Most of the large choroidal vessels run parallel to the meridians and to one another with the choroidal veins fanning outward from the point at which the vorticose veins penetrate the sclera (Figs. 21-5 and 21-6). Branches of these vessels form the middle-sized vessel layer, which leads to and empties the choroidal capillary layer, which in turn nourishes the outer layers of the retina.
The Eye
Development
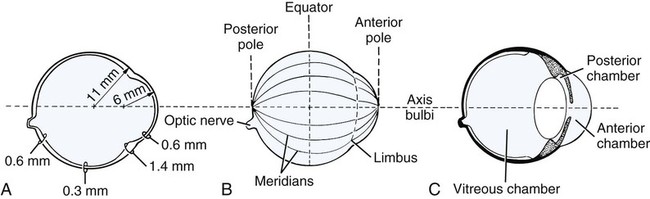
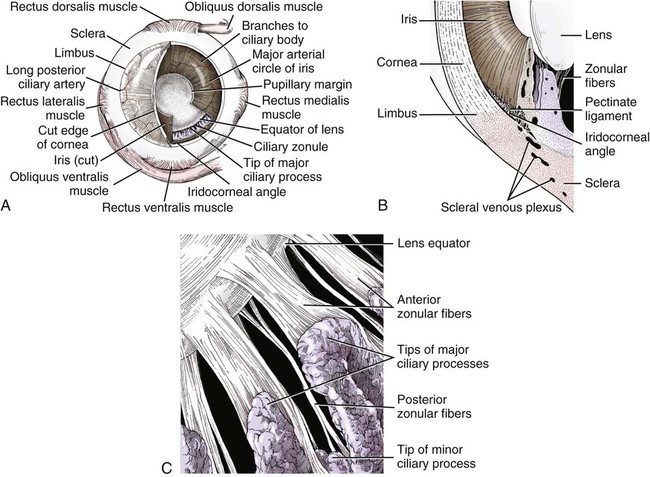
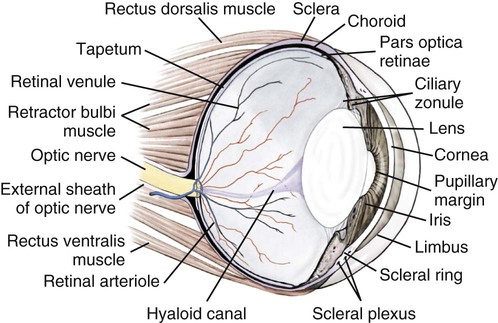
The Eyeball
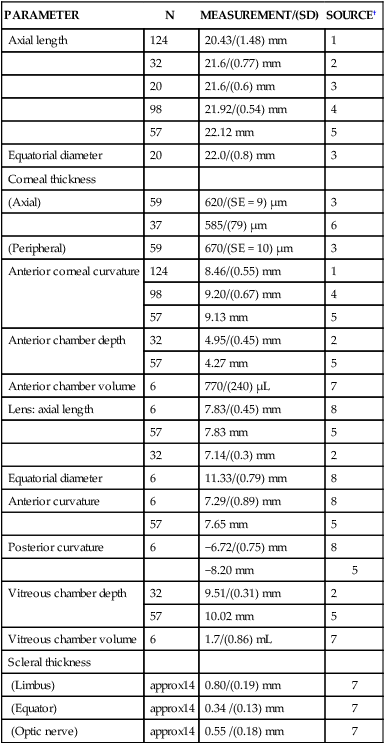
PARAMETER
N
MEASUREMENT/(SD)
SOURCE†
Axial length
124
20.43/(1.48) mm
1
32
21.6/(0.77) mm
2
20
21.6/(0.6) mm
3
98
21.92/(0.54) mm
4
57
22.12 mm
5
Equatorial diameter
20
22.0/(0.8) mm
3
Corneal thickness
(Axial)
59
620/(SE = 9) µm
3
37
585/(79) µm
6
(Peripheral)
59
670/(SE = 10) µm
3
Anterior corneal curvature
124
8.46/(0.55) mm
1
98
9.20/(0.67) mm
4
57
9.13 mm
5
Anterior chamber depth
32
4.95/(0.45) mm
2
57
4.27 mm
5
Anterior chamber volume
6
770/(240) µL
7
Lens: axial length
6
7.83/(0.45) mm
8
57
7.83 mm
5
32
7.14/(0.3) mm
2
Equatorial diameter
6
11.33/(0.79) mm
8
Anterior curvature
6
7.29/(0.89) mm
8
57
7.65 mm
5
Posterior curvature
6
−6.72/(0.75) mm
8
−8.20 mm
5
Vitreous chamber depth
32
9.51/(0.31) mm
2
57
10.02 mm
5
Vitreous chamber volume
6
1.7/(0.86) mL
7
Scleral thickness
(Limbus)
approx14
0.80/(0.19) mm
7
(Equator)
approx14
0.34 /(0.13) mm
7
(Optic nerve)
approx14
0.55 /(0.18) mm
7
Fibrous Tunic
Sclera
Cornea
Vascular Tunic
Choroid
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Eye
Only gold members can continue reading. Log In or Register to continue