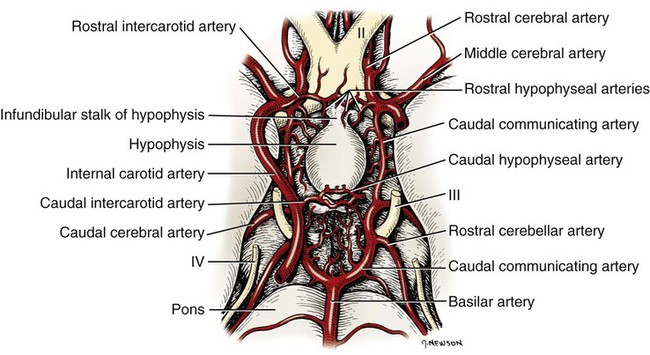
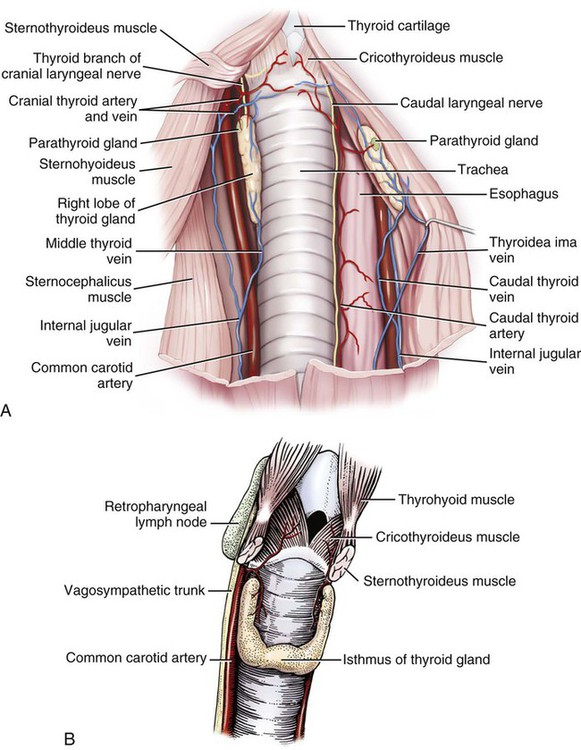
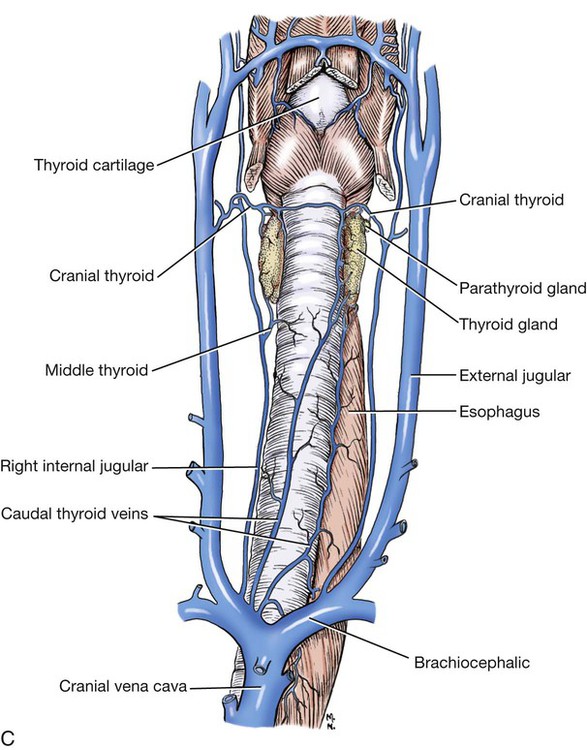
The significant and enduring role of the endocrine secretions also affects normal development, differentiation, and functioning of the immune system (Kelley et al., 1988), subtleties of sexual differentiation (Aumuller, 1983) and dimorphism, fertility control, alimentation, and normal growth and aging. A normally functioning endocrine control system is essential to the integration of the functioning body systems. A malfunctioning endocrine system causes dramatic changes in body form, function, and behavior. Diabetes insipidus, hypophyseal adenoma, diabetes mellitus, hypothyroidism, hyperthyroidism, interstitial cell tumor, and hyperadrenocorticoidism are a few of the major endocrine disturbances affecting the dog. Bloom (1959) presents a general discussion of endocrine diseases affecting the dog. The endocrine organs vary in structure and function among the breeds (Stockard, 1941), among individuals, seasonally, and at a cellular level diurnally. The morphologic characteristics of the endocrine organs can change rapidly in response to variations in normal physiologic activity. In many of the endocrine tissues there is a storage of precursors or products of cellular synthesis leading to the formation of the active hormone. The adrenal cortex, interstitial endocrine cells of the testis, and endocrine cells of the corpus luteum store neutral fats and cholesterol in large quantities. The thyroid produces great amounts of thyroglobulin and stores such materials in the follicular lumina. In light of this “reserve capacity” and the normal variation in cell numbers or percentages within the same individual, abnormal changes leading to or resulting from disease of the endocrine system are very difficult to determine by morphologic means. Such findings suggestive of hyper- or hypofunctioning must be correlated with a case history, clinical signs, and assays for hormone production. Hormonal assay is accomplished primarily by direct measurement of blood hormone levels or urinary excretion of hormone metabolites. The dog continues to serve as a model for endocrine research and, as such, has provided much of the comparative information relating to the functions of this system. The term hormone was actually first applied by Bayliss and Starling (1902) in their observations of the mechanisms of pancreatic secretion in the dog. In 1922, when Banting and Best made medical history by discovering the effects of pancreatic extract in reducing blood sugar, the dog was the experimental animal. The dog was also the test animal used for the demonstration of secretin (Ivy & Oldberg, 1928; Kosaka & Lim, 1930). Michaelson (1970) presented a review of the general anatomic features of the endocrine glands of the dog, along with an account of specific functional and clinical parameters. Much of the structural and functional detail relative to the endocrine system of the dog has been inferred from work with other mammals, including humans, and specific information about the morphology of the dog endocrines is still widely scattered in the literature. Stockard (1941) presented detailed information on the morphologic characteristics of the endocrines in many breeds of dogs. Venzke (1976) discusses the macroscopic anatomy of selected endocrine organs of the dog. Compendia have been edited by Harris and Donovan (1966) for the pituitary gland; Pitt-Rivers and Trotter (1964) for the thyroid gland; Chester-Jones (1957), Nussdorfer (1986), and Chester-Jones et al. (1986) for the adrenal cortex; and Chester-Jones (1976) and Epple et al. (1990) for comparative endocrinology. A terminology appropriate to the gland follows and is based on the Nomina Anatomica Veterinaria (2005), Nomina Histologica (1992), and Nomina Anatomica (1989) (Figs. 10-1 and 10-2). The hypophysis, or pituitary gland (glandula pituitaria), is a reddish appendage attached at the ventral midline to the diencephalon (Fig. 10-1). The Greek term hypophysis cerebri conveys this positional meaning, hypo meaning under and physis meaning growth—thus the growth on the undersurface of the brain. The term pituitary is derived from a historical interpretation by Vesalius concerning the function of this gland as the source of nasal exudate, pituita, or phlegm (Field & Harrison, 1957). The size of the hypophysis varies greatly among breeds of dogs and among individuals of the same breed (Hanström, 1966; Hewitt, 1950; Latimer, 1941, 1965; Stockard, 1941; White & Foust, 1944). In the adult of the mesaticephalic breeds, the size of the unpreserved gland is approximately 1 cm in length, 0.7 cm in width, and 0.5 cm in depth. Its weight is approximately 0.06 g in the male. The hypophysis of the larger dog shows an absolute increase in size, but a relative decrease in proportion to body weight. When other factors such as breed and nutrition are constant, the hypophysis of the female is somewhat larger than that of the male. It is also larger in the gravid female than in the nongravid female (Latimer, 1941; White & Foust, 1944). Although small, this organ plays a major regulatory role in the entire endocrine system. The close structural positioning of the glandular and nervous parts of this gland is symbolic of its function in interrelating the nervous and endocrine systems. So extensive are the influences of the hypophysis on cells, tissues, and organs, that it is often referred to as the “master gland” of the body. The early work of Putnam et al. (1929), which reported the effects of administering extracts of the gland to young dogs, demonstrated the widespread changes in body tissues caused by injections from the “master gland” and indicated the significance of this endocrine gland. The works of Crowe et al. (1910) and Dandy and Reichert (1925) established that the hypophysis was essential for the maintenance of life. The hypophysis occupies a bony recess in the basisphenoid (os basisphenoidale) (Fig. 4-18). The recess is a shallow, oval depression, the hypophyseal fossa (fossa hypophysials). The rostral and caudal margins of the fossa are formed by the rostral clinoid processes and the dorsum sella, respectively. When the fossa is viewed dorsally, the rostral and caudal clinoid processes accentuate the boundaries of the fossa on the dorsal surface of the basisphenoid bone referred to as the sella turcica. In the dog the fossa is quite shallow and is lined by the external, or endosteal, layer of dura mater. The inner, or meningeal, layer of the dura forms the diaphragma sellae. The latter does not pass directly into the fossa with the external dural layer but extends partially over the dorsal aspect of the fossa to provide an incomplete septum. The primary attachments of this septum, or diaphragm, are by way of the clinoid processes. A large oval foramen is present in the center of the diaphragm, which loosely encircles the stalklike connection of the hypothalamus to the hypophysis as it passes into the fossa. This thin meningeal layer then continues around the main portion of the gland as a delicate capsule. Schwartz (1936) demonstrated that the subarachnoid space does not invest the hypophysis. The space created by the separation between the inner and outer dural layers contains prominent cavernous and intercavernous sinuses. Large cavernous sinuses bound the hypophysis laterally and are connected by intercavernous sinuses. The larger intercavernous sinus passes just caudal to the hypophysis. The smaller intercavernous sinus is present in only some individuals and, when present, it passes rostral to the hypophysis. The proximal portion of the middle meningeal artery and the anastomotic ramus of the external ophthalmic artery lie within each of the cavernous sinuses. The internal carotid artery also courses through each of the cavernous sinuses on the lateral margins of the hypophysis, from the dorsum sella rostral to the region of the optic chiasm. The oculomotor, trochlear, and abducent nerves and the ophthalmic nerve from the trigeminal nerve pass in close proximity to the hypophysis. The interpeduncular cistern is adjacent to the caudal aspect of the attachment of the hypophysis to the hypothalamus, and within the cistern lies the caudal part of the cerebral arterial circle (circulus arteriosus cerebri). A bony wall separates the hypophyseal fossa from the sphenoid sinus, which lies rostroventral to it. The positional relations of these structures to the hypophysis create a degree of surgical risk and, as reported by Harrison (1964) and emphasized by Farrow (1969), may account for many of the signs accompanying hypophyseal disease. As noted by Hanström (1966), much of the literature demonstrates individual and breed differences in the morphologic characteristics of the hypophysis. The hypophysis of the adult (Figs. 10-1 and 10-2) has grossly visible rounded protuberances, the adenohypophysis and the neurohypophysis. The adenohypophysis, being composed of glandular parenchyma and having an extensive blood supply, appears reddened and friable in comparison with the pallor and the brainlike texture of the neurohypophysis. A median section of the hypophysis and the hypothalamus, when examined with slight magnification, reveals further subdivisions of the organ (Fig. 10-1). The gland is suspended from the midline of the hypothalamus by a cylindrical stalk. This stalk is an extension from the tuber cinereum of the hypothalamus. It is the proximal portion of the neurohypophysis, the infundibulum. In most dogs the third ventricle continues as an invagination into the infundibulum, a recess called the pars cava but it rarely passes into the more distal portion of the infundibulum. The distal compact infundibulum is continuous with the distal enlargement, the neural lobe (lobus nervosus) of the neurohypophysis, which is the modest expansion at the distal end of the infundibulum and the major portion of the neurohypophysis. By the 7-mm stage of development in the dog, a small portion of oral ectoderm lining the dorsum of the stomodeum contacts the ventral surface of the neural tube. This contact or adhesion between oral and neural ectoderm is maintained while differential growth and the resultant proliferation of mesoderm continues in the head region. The neural ectoderm retains its relative position, and the adjacent portion of oral ectoderm is drawn away from the stomodeum, first as a cul-de-sac and then as a closed vesicle, saccus adenohypophysialis, separated from the developing oral cavity. With continued differentiation of the neural ectoderm, a small projection or evagination develops at the midline of the ventral surface of the diencephalon at the point of adhesion to the oral ectoderm. This structure, the sacculus infundibuli, becomes surrounded by the collapsing vesicle of oral ectoderm. The adjoining mesenchyme develops the stroma and vascularization for this parenchymal primordium of the hypophysis (Herring, 1908b; Kingsbury & Roemer, 1940; Latimer, 1965; Stockard, 1941). The general microscopic features of this gland are those of an endocrine gland and a segment of central nervous system tissue. The stroma is formed by a capsule of delicate pial connective tissues that forms around the neurohypophysis during development and remains as a boundary between it and the adenohypophysis subdivisions. The adenohypophysis is enveloped by a delicate investment of collagenous connective tissue of the arachnoid that binds the adenohypophysis to the adjoining inner layer of dura mater. At a point representing the original connection to the oral ectoderm on the midventral surface of the adenohypophysis, the adenohypophysis is attached to the inner dural layer, which is fused to the outer layer. This attachment is easily separated and usually accounts for some separation artifact in preparations of the pars distalis adenohypophysis. When a small remnant of development called the parahypophysis is present, it is attached at this location (Kingsbury & Roemer, 1940). The stroma that forms a capsule for both the neurohypophysis and the adenohypophysis is originally quite delicate and increases in amount only slightly with advancing age. The blood vessels are invested by small amounts of adventitial connective tissues, and the parenchymal cells of the adenohypophysis are supported by a delicate interstitium of reticular fibers. The pars distalis adenohypophysis has a parenchyma of epithelioid cell types arranged in small, interconnected clusters, racemi endocrinocyti, that are bounded and permeated by numerous sinusoids. These aggregates of cells are quite varied in size and shape and are arranged three-dimensionally as anastomosing lattices. The close proximity of nearly all parenchymal cells to a sinusoid is maintained throughout. By the use of specific staining techniques, especially immunohistochemistry, cellular biochemistry and structure have been related to the specific hormonal secretions of these cells. With routine staining there is a separation of three distinctive cell populations: endocrinocytus acidophilus, endocrinocytus basophilus, and endocrinocytus chromophobus. The frequency of occurrence of each cell type varies according to the age, sex, breed, and physiologic state. Francis and Mulligan (1949) reported that in the adult male dog the acidophilic cells outnumbered the basophilic cells approximately five to one and that the chromophobic cells occurred with three times the frequency of the basophilic cells. Stockard (1941) reported that the basophilic cells were outnumbered 30 to 1 by the acidophilic cells. White and Foust (1944) reported no significant sex differences and a ratio of 11 to 1, acidophilic to basophilic cells. The basophilic endocrine cells possess cytoplasmic granules that have a moderate affinity for the basic component of routine laboratory stains. The granules are composed of glycoprotein and react positively when stained with dyes specific for that component. The cells are larger than the acidophilic cells, measuring approximately 20 µm in diameter, and are more elongated. Like the acidophilic cells, these cells are generally observed along a sinusoid. They occur in greater numbers at the periphery of the pars distalis adenohypophysis. Stockard (1941) has reported that basophilic cells proliferate during proestrus. Thyrotrophin and the gonadotrophins are the hormones produced by specific basophilic cell types. The chromophobic cells do not stain well with routine dyes. They occur in moderate numbers near the central region of a racemose. Their nuclei are easily visible, but their cytoplasmic boundaries are difficult to determine. There is equivocal evidence that some of these cells, which lack marked staining affinity, produce adrenocorticotrophin (Goldberg & Chaikoff, 1952; Mikami, 1956; Purves, 1966). Ricci and Russolo (1973) present immunocytologic evidence that suggests this hormone is produced by the chromophils. Many investigators have described a fourth cell type in the dog. Most believe it to be a functional variant (Hartman et al., 1946; Purves & Griesback, 1957; Smith et al., 1953; Wolf & Cleveland, 1932). Kagayama (1965) and Gale (1972) term this cell a stellate, or follicular cell. Goldberg and Chaikoff (1952), Carlon (1967), and Gale (1972) describe six cell types based on specific histochemistry or structural features. The parenchyma of the pars intermedia adenohypophysis is a complexly folded pseudostratified columnar epithelium. It envelops nearly all of the neural lobe of the neurohypophysis and is separated from it by only a delicate band of vascularized, pial connective tissue. The remnant of the vesicular space, the hypophyseal cavity, separates it from the pars distalis adenohypophysis. The pars intermedia adenohypophysis blends with the pars distalis adenohypophysis as it reflects distally to join with that part. Where it is continuous with the pars tuberalis adenohypophysis, the folded epithelium projects into the lumen of the hypophyseal cavity. These folds are more prominent in the brachycephalic breeds. Infrequently, there are villuslike projections extending for short distances into the neurohypophyseal tissues. The parenchyma is normally devoid of sinusoids but has access to numerous pial capillaries at the neurohypophyseal surface. Often these vessels can be seen projecting into the connective tissue septa of the folds. The epithelioid cells stain only faintly. Melanocyte-stimulating hormone and adrenocorticotropin are secreted by these cells (Halmi et al., 1981). Cells of the neural lobe and the infundibulum are chiefly glial cells, which are termed gliocyti centrales (pituicytes). These cells support axons in an extensive neuropil. These axons are processes of neuronal cell bodies found in the nuclei of the hypothalamus and compose the supraopticohypophyseal and paraventriculohypophyseal tracts (Fig. 10-1). From these processes are released neurohumors produced in the hypothalamus and termed vasopressin and oxytocin. These neurohumors enter the systemic circulation. The tuberohypophyseal tracts convey releasing factors from numerous hypothalamic nuclei to the tuber cinereum and infundibulum where they enter the capillary loops that circulate to the adenohypophysis. The arterial supply of the hypophysis arises from two major sources, the internal carotid arteries and the caudal communicating arteries (Fig. 10-3). Several branches passing directly to the region of the gland arise from the rostral and caudal intercarotid arteries and the caudal communicating arteries. The number of vessels from these sources that converge on the hypophysis is extensive and was described by Dandy and Goetsch (1910) as appearing “like spokes to the hub of a wheel.” The rostral intercarotid artery provides a variable number of branches, 4 to 10, to the region of the infundibulum (Basir, 1932). An equally extensive group of vessels arises from the rostral communicating arteries. In addition, a vessel arises from each of the internal carotids and proceeds toward the infundibulum. Less prominent vessels may also originate from the caudal aspect of the arterial circle. All of these vessels pass centripetally toward the infundibulum where they join in forming a plexus, the mantle plexus (Green, 1951). The plexus is incorporated into the meningeal investment of the hypophysis. From this mantle plexus many arterioles enter the tuber cinereum and provide capillaries to the primary blood capillary network (rete hemocapillare primarium). On the rostral and lateral surfaces of the tuber cinereum major vessels arise from the mantle plexus or as direct branches of the intercarotid vessels that are termed the rostral hypophyseal arteries. Several of these also provide capillaries to the tuber cinereum and the initial portion of the infundibulum and join in the primary blood capillary network (Akmayev, 1971a). These capillaries receive neurohumoral secretions called releasing factors, which are subsequently carried from this region of the hypothalamus, called the median eminence gland by Reichlin (1974), to the hypophysis via a portal blood vascular system (Campbell, 1970; Green, 1966). Akmayev (1971b) reported that those nuclei of the tuber cinereum that have the greatest vascularity have the smallest caliber capillaries. A major portion of the venous drainage from this capillary network returns to the surface of the infundibulum and is collected by veins that run parallel to its outer surface. These veins supply the sinusoids of the adenohypophysis. The capillaries (sinusoids) of the adenohypophysis form the secondary blood capillary network (rete hemocapillare secundus). The veins that connect these two capillary networks are the hypophyseal portal vessels (venulae portis hypophysis), meaning the gateway vessels to the gland. This portal circulation in the dog is less obvious than in some other species owing to the relatively short infundibulum in the dog. Green (1966) and Bergland and Page (1979) suggest a less direct influence of the circulation. The regulation of the adenohypophysis by the hypothalamus is made possible by the architecture of the portal system. Structural and functional data suggest that blood entering the hypophysis from these multiple sources will eventually percolate through the sinusoids of the pars distalis adenohypophysis. From these sinusoids venous drainage is by way of vessels that exit from the gland parenchyma and empty into the cavernous and intercavernous sinuses (Dandy & Goetsch, 1910; Flerko, 1980; Green, 1951, 1966; Morato, 1939). The parenchyma of the neurohypophysis is primarily composed of axons that extend from cell bodies in the hypothalamic regions (Dandy, 1913; Watkins, 1975). The majority of these cell bodies are located in the supraoptic and paraventricular nuclei and, to a lesser degree, in other hypothalamic regions (Green, 1951). These axons extend into the neural lobe in the supraopticohypophyseal and paraventriculohypophyseal tracts. These are unmyelinated axons that convey and release a neurosecretory product. The nerve supply to the hypophysis passes primarily to the neurohypophysis Sympathetic axons arising from the cranial cervical ganglion pass by way of the tunica externa of the internal carotid artery and its branches to the vessels of the hypophysis (Dandy, 1913; Green, 1951; Truscott, 1944). A parasympathetic innervation has not been described. The results of ablation experiments suggest that hormonal output from the adenohypophysis is not under direct autonomic control. Yamada et al. (1956) and Green (1966) review the literature on innervation to portions of the adenohypophysis. Castel et al. (1984) provide a comprehensive review of neurosecretory systems and of the neurohypophyseal tracts specifically. The thyroid tissue in most dogs forms paired structures, although each gland, glandula thyroidea, is referred to as a lobe. It is an elongated, dark red mass attached to the external surface of the cranial portion of the trachea (Fig. 10-4). It is positioned laterally and somewhat ventrally on the trachea, spanning the initial five to eight tracheal rings on its respective side. The size of the thyroid is variable, depending on the breed and individual (Marine, 1907; Stockard, 1941). In the adult of the medium-sized breeds, the fresh gland is approximately 5 cm in length, 1.5 cm in width, and 0.5 cm in thickness, with the dorsal margin of the gland being somewhat thicker than its ventral counterpart. The thyroid is the largest of those ductless glands that perform only an endocrine function. By virtue of its size and position, the thyroid is unique among the exclusively endocrine organs in that it can be palpated during a physical examination, especially when enlarged. The weight of the fresh gland of the adult is quite variable. Data comparing the thyroid mass to body weight revealed a ratio of approximately 0.1 g thyroid per kg body weight (Gilmore et al., 1940; Mulligan & Francis, 1951). The thyroids exert a major control on the metabolic processes of the body and affect most body systems. The thyroid hormone is synthesized by the glandular parenchyma, stored intercellularly, and released from the gland into the circulation. Foster et al. (1964a, b) demonstrated the presence of a second hormone produced by the thyroid called thyrocalcitonin, or calcitonin, which lowers blood calcium by stimulating calcium uptake by the skeleton. The parenchyma of the thyroid develops from structures of the pharyngeal endoderm. Beginning at the 4-mm stage, the midventral surface of the pharynx gives rise to a tissue thickening, the thyroid diverticulum (Godwin, 1936). Further growth in size occurs predominantly at the distal end of the diverticulum. Its connection to the pharynx narrows to become a stalk before separating completely. If a connection persists, it may appear as a duct, the thyroglossal duct, with functional glandular epithelium and numerous cysts along its course. After separating from its attachment to the pharynx, the median thyroid diverticulum forms a bilobed mass connected by a broad isthmus, oriented transversely on the ventral aspect of the trachea. As the primordium shifts caudally with morphogenesis of the head and neck, it passes near the developing pharyngeal pouches. A ventral component of the fourth pharyngeal pouch epithelium fuses with the median thyroid mass (Boyd, 1964; Godwin, 1937a). These components continue their development as a single unit, and any subsequent recognition of the contributing components is impossible. The isthmus narrows and in many cases remains only as a fibrous band connecting the caudal aspect of each gland. The orientation of each gland eventually parallels the trachea. The stroma of the glands is developed from the surrounding mesoderm. An outstanding feature of thyroid development in the dog is the frequent occurrence of accessory thyroid tissue (glandula thyroidea accessoria). Islets of the rapidly proliferating cells of the thyroid primordia separate from the main mass and become incorporated in the developing structures of the neck and thorax. Functional accessory thyroid tissue is frequently found along the trachea, at the thoracic inlet, within the mediastinum, and along the thoracic portion of the descending aorta. Swarts and Thompson (1911) reported accessory thyroid tissue in the pericardial sac of 24 of 30 dogs examined. Halsted (1896), French (1901), Godwin (1936), and Kameda (1972) reported on the occurrence of numerous accessory thyroid tissues in the dog, and Smithcors (1964) reported some accessory tissues in all embryos and nearly one-half of adults examined. As the main mass of developing thyroid parenchyma extends first ventrocaudally and then laterally, it incorporates cells that in the adult will function to produce calcitonin (thyrocalcitonin). The origin of these cells has long been considered pharyngeal endoderm of the fourth pharyngeal pouch. These cell groups of the developing neck, ultimobranchial bodies, fuse with and become dispersed within the developing thyroid parenchyma (Godwin, 1937a). As thyroid development proceeds, these cells occupy positions satellite to and between thyroid follicles (Nonidez, 1932a, b) and differentiate to form the parafollicular endocrine cells (endocrinocyti parafolliculares) and the interfollicular endocrine cells (endocrinocyti interfolliculares), respectively. Evidence presented by Pearse and Polak (1971) suggests that the ultimobranchial bodies are invaded by cells of the neural crest prior to their migration into the developing thyroid. They suggest a neural crest rather than pharyngeal endoderm as the tissue of origin for the parafollicular and interfollicular cells. Some interfollicular tissue forms interfollicular islets (insulae interfolliculares), which are believed to be stem cells for the formation of new thyroid follicles.
The Endocrine System
General Features of the Endocrine Glands
The Hypophysis
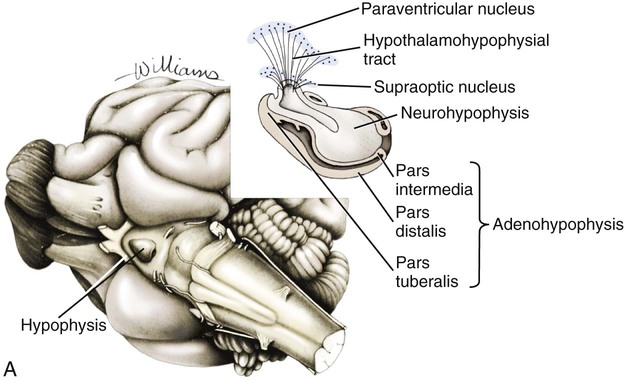
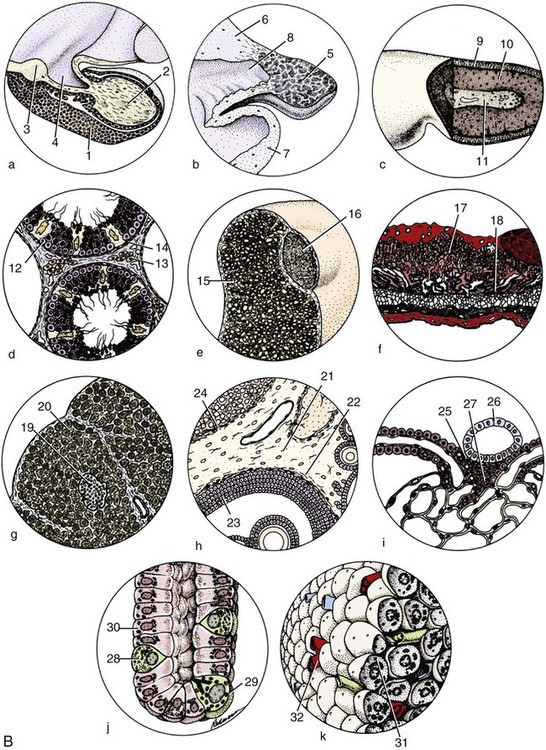
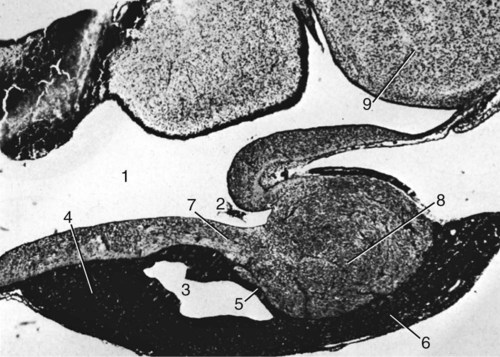
Macroscopic Features
Mesoscopic Features
Developmental Anatomy
Microscopic Features
Vascularization
Innervation
Thyroid Gland
Developmental Anatomy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree