CHAPTER 36 The Digestive System*
Prenatal and Neonatal Development
In later life, the ability to change digestive function varies among species in accordance with natural variation in the diet. The cat, which is an obligate predator, is less able to vary its pancreatic and GIT enzyme activity than is the more omnivorous dog. Dogs also possess a tremendous capacity to adapt their GIT function over an enormous range of energy requirements. For most dogs, the largest demand on the GIT for processing nutrients occurs during growth. A weaned puppy may require 2 to 3 times the energy per unit body weight of a sedentary adult dog.*
The Salivary Glands
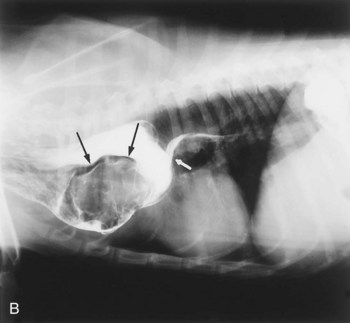
A sialocele is a collection of saliva in tissue. Any age, sex, and breed of dog or cat can be affected. Sialocele formation most commonly involves the sublingual and mandibular salivary glands, where blockage of a duct or rupture of the gland causes extravasation of saliva into the surrounding connective tissue. The saliva may gravitate to the sublingual area (often referred to as a ranula), intermandibular space, mediastinum, or pharyngeal area (Figure 36-1). Swelling may develop suddenly but is more likely to be in the form of a slowly developing, fluctuant sac. Diagnosis of sialocele is based on physical appearance of the cervical swelling and on cytologic evaluation. Needle aspiration of the swelling yields a viscous, mucoid fluid that is usually clear or brown. Total excision of the affected gland, which is usually the sublingual or mandibular gland, and drainage of the sialocele provide the only long-term therapy. Removal of an elliptic portion of the wall of a ranula allows direct drainage of saliva into the oral cavity. The surgical techniques for the management of salivary gland disorders are described elsewhere.
The Esophagus
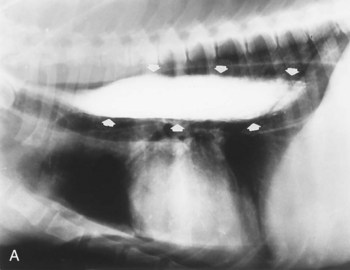
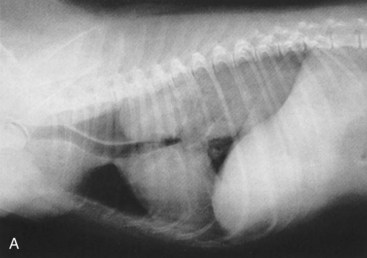
Signs caused by the vascular ring anomalies result from esophageal entrapment and obstruction, with subsequent precordial megaesophagus. When weaning to solid food, puppies and kittens will repeatedly regurgitate. Although regurgitation is usually associated with eating when solid food is first fed, it will occur at variable times after eating as the precordial megaesophagus worsens. Occasionally signs in the puppy or kitten are associated with ingestion of maternal milk. Affected animals are obviously malnourished and underweight. The diagnosis of a vascular ring anomaly-induced megaesophagus can usually be confirmed by a positive contrast esophagogram (Figure 36-2).
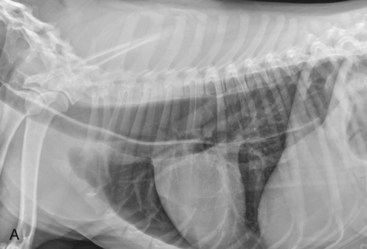
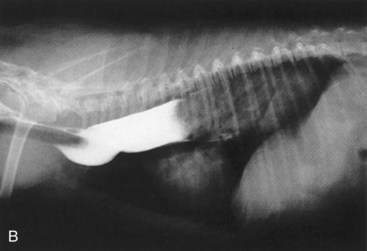
Survey thoracic radiographs consistently reveal a dilated esophagus (Figure 36-3). Evidence of aspiration pneumonia is frequently present and consistent with the signs of dysphagia and regurgitation.
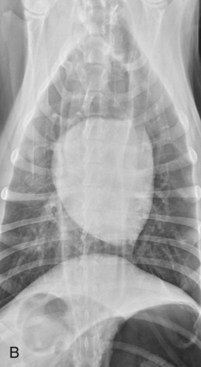
When the esophagus is not observed visually on survey radiographs, a contrast esophagogram is required. A barium contrast study better defines the degree of esophageal dilation, lack of function, and extent of involvement (Figure 36-4). The study helps rule out congenital vascular ring anomalies or other causes of localized obstruction that might contribute to megaesophagus and outlines the funnel shape of the caudal region of the esophagus to rule out invasive processes that may cause irregular or asymmetric narrowing.
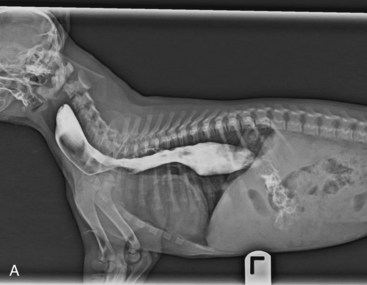
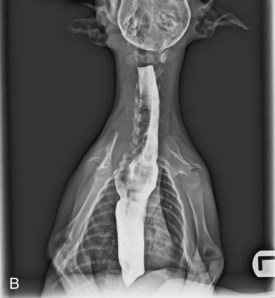
Figure 36-4 Megaesophagus in a puppy. A and B, A two-view contrast study demonstrating the extent of the dilation.
(Courtesy John Feleciano.)
Disorders of the Gastroesophageal Junction
Gastroesophageal intussusception or invagination involves the telescoping of all or part of the stomach into the esophageal lumen (Figure 36-5). Occasionally the spleen and pancreas are also included. The cause of this condition is not understood, but an incompetent gastroesophageal junction must be suspected. Gastroesophageal intussusception generally occurs in puppies of large breeds and in kittens, particularly those with congenital megaesophagus. A puppy or kitten will be presented to the veterinarian with sudden onset of difficulty in breathing, impending shock, and a history of vomiting. The sudden onset of signs and radiographic appearance of the mass are the keys to the diagnosis. Treatment is surgical reduction of the intussusception and a gastropexy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree