CHAPTER 32 The Cardiovascular System
Screening for Heart Disease
Classification of Congenital Heart Disease
Lesions Producing Left Basilar Murmurs
Aortic Stenosis
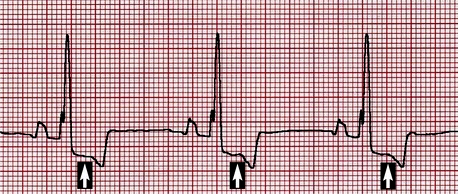
Although nonspecific, electrocardiographic changes that may accompany SAS include increased R wave amplitude and QRS duration consistent with left ventricular enlargement, as well as ST segment and T wave alterations suggestive of myocardial ischemia (Figure 32-1). VPCs may be evident on baseline electrocardiography (ECG), and studies of Holter examinations suggest the overall number and grade of VPCs display a modest correlation with pressure gradient. Ventricular tachycardia and ultimately fibrillation are the presumed mechanism for sudden death in dogs with SAS.
Pulmonic Stenosis
In cases of moderate to severe PS, the ECG almost always displays criteria for right ventricular enlargement (Figure 32-2). The P waves are usually normal. Ventricular arrhythmias appear to be less common than in dogs with SAS.
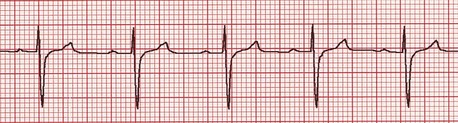
Figure 32-2 Lead II, 5 mm/mV, 50 mm/s. Deep S waves frequently accompany dogs with moderate to severe pulmonic stenosis.
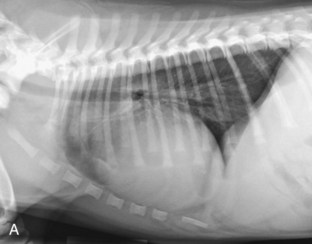
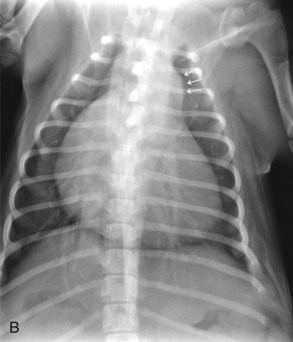
Radiography usually reveals moderate cardiomegaly with a right heart enlargement pattern (Figure 32-3). Poststenotic dilation of the main pulmonary artery produces loss of the cranial cardiac silhouette on the lateral view and dilation at approximately the 2 o’clock position on the dorsoventral (DV) view.
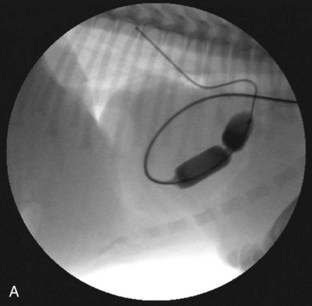
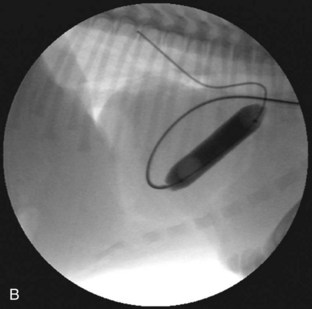
Several techniques have been described and are available for the treatment of PS. Valvular PS with a normally developed pulmonary annulus is most often treated by percutaneous balloon valvuloplasty (Figure 32-4). The goals of intervention include improving survival and resolving clinical signs in symptomatic animals. Surgical success, defined as a reduction of the pressure gradient into the mild category, or in markedly severe cases as a reduction in the pressure gradient of more than 50%, is achievable in up to 80% of dogs. Valvuloplasty has been reported to reduce clinical signs and mortality compared with animals not undergoing surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree