Chapter 38 The California Condor (Gymnogyps californianus)
Veterinary Program: 1997-2010
The California condor recovery program has experienced tremendous growth in the decade since Ensley’s report,10 which included a thorough and comprehensive review of biologic data, captive management and husbandry, transportation, quarantine procedures, clinical techniques, annual examination, radiographic imaging, surgery, reproduction, medical conditions of chicks at hatch, gender determination, pathology, environmental contaminants affecting condor health, bacterial pathogens, fungal diseases, parasitology, clinical evaluation of a sick condor, and medical treatments for released condors through March of 1997. The aim of this chapter is not to reproduce this information, but to update it with new information. Also presented is an expanded section on free-ranging condors.
Clinical Considerations
Normal hematologic values have been published.9 California Condors at the Los Angeles Zoo are now restrained in a catch cage to allow for much improved capture, rather than attempting capture from large free-flight aviaries. This is highly recommended as a safer and less stressful way to capture birds.
Annual Examination, Bacterial Pathogens, and Parasitology
Captive adult or subadult birds may be examined annually, biannually, or opportunistically, depending on the facility. Recommended laboratory examinations include complete blood count (CBC) and complete chemistry panel, electrophoresis, and bile acid determination for captive birds that appear healthy. The volume of blood obtained is determined by the clinician at the institution based on laboratory needs. Two valuable female breeding birds with egg yolk peritonitis were radiographed and diagnosed solely because of abnormalities in electrophoretic patterns during routine examinations at the Los Angeles Zoo; both recovered well from surgery and resumed breeding with no further problems. It is recommended that valuable breeders be examined annually if this may be done safely. Routine fecal cultures are no longer considered to be necessary because no pathologic disease process has been seen in adult birds related to bacteria considered to be pathogenic to other species. Lead and zinc testing procedures are also unnecessary in captive birds with no history of metal exposure or ingestion. Additionally, Aspergillus testing is not necessary in normal adults and chicks (see fungal section). Serum banking is performed at most institutions, and strongly recommended. The use of a Vacutainer butterfly (19- to 21-gauge needle, according to the size of vessel, largely dependent on the bird’s temperature) is a fast and efficient way to obtain blood samples, because normal birds have a high enough blood pressure to fill large tubes easily. Annual fecal examinations for parasites are performed at some institutions and recommended. Strongyle nematode eggs have been seen and successfully treated at the Los Angeles Zoo. Coccidia has also been seen at more than one institution, without clinical signs.30
Clinical Techniques
Physical Restraint
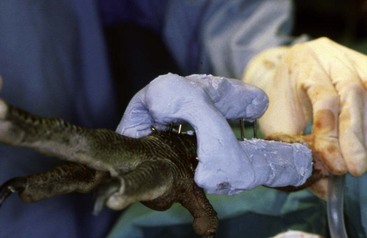
Experienced handlers may hand-grab birds without using a net or, using condor techniques that relate to condors’ intelligence, perceptive nature, and behavioral response to displays, restraint with a net may be accomplished with minimal stress. Operant conditioning and slow methodic methods have been very successful in decreasing stress and capture-related injury.7 Also, if experienced, only one person may be needed for restraint (Fig. 38-1).
Care must be taken if the bird is held at the base of the skull not to press into the ventral neck. One case of postrestraint tracheal hemorrhage has been seen, likely because of improper restraint of the neck (the bird recovered without incident). An improved technique is to hold the bird by the beak only and push the head back toward the body. The bird cannot strike out this way, and does not struggle to do so (Fig. 38-2).
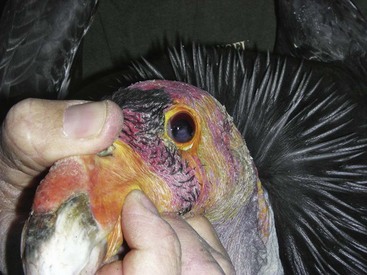
Figure 38-2 Proper beak restraint and finger placement. This bird has an iris mass seen in the left eye.
(Courtesy J. Wynne.)
Oral Medication Administration and Oral Examination
Because of the strength of the beak, inserting fingers at the commissures of the beak allows an oral examination to be performed without risk of being bitten (see Fig. 38-2). Oral medication in chunks of meat may be force-fed in a similar manner by experienced handlers.30
Intravenous and Intraosseous Access
Although clinicians have used the jugular vein and ulnar vein for venipuncture, the medial metatarsal vein is the easiest IV access and location for maintaining an indwelling IV catheter. Intraosseous catheters are usually not needed because of the availability of this vein but, in one case, an intraosseous ulnar catheter was used for fluid administration and the bird suffered a fatal pulmonary edema. Further investigation showed the ulna to be pneumatized, with pneumatic foramina at the proximal and distal ends of the bones in this species (as well as in Andean condors and other species of storks and vultures examined).23 One dissection has shown that the tibiotarsus may be a better location because it was the only marrow-filled long bone found,30 but to date this has not been clinically attempted.
Surgery
Numerous surgeries have been performed on this species, including orthopedic surgeries (Fig. 38-3) and ventriculotomies (see later). This species exhibits remarkable postsurgical healing and recovery abilities. Patagial tears caused by improper wing transmitter placement have been seen that required surgical repair, but severe tears that had torn completely through the patagium were not reparable.30
Reproduction and Medical Conditions of Chicks at Hatch
Captive chick rearing methods have been detailed.13 Intervention during hatching, if needed, has been detailed10 and is routinely performed at most institutions. Egg yolk infections have been successfully treated by surgically removing the affected yolk, along with medical treatment. At the Los Angeles Zoo, a standard dose of ceftriaxone, 12.5 mg SC two to four times daily, is used for newly hatched chicks. The drug is applied to the shell membrane in chicks in the final days of development in an opened egg.
Pathology
Pathologic studies continue to be performed for the entire population by the Zoological Society of San Diego, with the exception of several birds that were suspected to have been killed in the wild, and necropsies were performed by the government for legal purposes. Mortality data for released birds from the beginning of the release program until 2002 have been published.25 A complete review of released bird mortalities is in process.22 After initial mortalities from electrocution and/or trauma from hitting power lines in California decreased because of experience and/or power pole aversion training, lead poisoning emerged as the most common single cause of mortality in free-flying released birds. Lead poisoning is the most frequent cause of death for free-flying wild birds in Arizona. Additional mortalities have been seen from trash ingestion (including zinc toxicity from pennies) in wild-hatched chicks, trauma and accidents (e.g., predators, conspecifics, rattlesnake bite, fire, accidental rope strangulation, drowning, fires), gunshot or arrow shot, ethylene glycol toxicity, and inanition and/or dehydration from failure to adapt. In captivity, causes of death for adults have included rattlesnake bite, trauma (primarily neck trauma caused by hitting fences; one case of head trauma likely caused by hitting the top of a crate), pulmonary edema secondary to an intraosseous catheter placed in the ulna, two deaths from unknown viral cause, and one death from West Nile virus. Poxvirus, likely from local wild birds, caused death in one chick, and various hatching malpositions and infections, West Nile virus, and parental trauma have been the causes of death in other chicks in captivity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree