Chapter 27
The Bone Marrow
The islands of hematopoietic tissue are composed of the following: erythroid, granulocytic, monocytic and thrombocytic series cells; marrow structural cells (adventitial cells); fat cells; and a few macrophages, lymphocytes, plasma cells, and mast cells. A general guideline on cellular location in bone marrow is as follows: Immature granulocytes are paratrabecular and perivascular, whereas mature granulocytes are central (interstitial); megakaryocytes and erythropoietic islands are adjacent to sinuses; plasma cells and mast cells are perivascular; and lymphocytes are dispersed interstitially with perivascular lymphoid aggregates. Hematopoietic tissue islands are bounded by adventitial cells, basement membrane, and endothelium lining the vascular sinuses (Figure 27-1). Mature hemic cells pass transcellularly through sinusoidal endothelial cells into the circulation.1–3
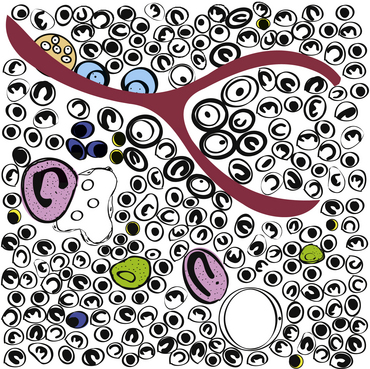
Figure 27-1 Schematic of hematopoietic bone marrow.
The islands of hematopoietic tissue are composed primarily of erythroid, myeloid, and megakaryocytic cells. The hematopoietic cells are surrounded by a network of vascular sinuses, stromal tissue, and extracellular matrix. Myeloblasts are paratrabecular (adjacent to bone), whereas late-stage granulocytes are central (interstitial); megakaryocytes (pink) and erythropoietic islands (immature red cells surrounding macrophages [green]) are often adjacent to sinuses; plasma cells (dark-blue) and mast cells are perivascular; and lymphocytes (yellow) are dispersed interstitially (note two small lymphocytes adjacent to the cluster of three plasma cells). Osteoclasts (orange) and osteoblasts (pale-blue) line the trabecular bone (red-brown). (Drawing by Cari Grindem-Corbett.)
Indications
The most common indication for bone marrow examination (either an aspiration biopsy or a core biopsy) is recognition of hematologic abnormalities not readily explained by a detailed history, physical examination, chemistry profile, and other clinical procedures. Bone marrow examination is often rewarding in evaluating animals with nonregenerative anemia, persistent neutropenia, or persistent thrombocytopenia.4–6
Bone marrow evaluation may also be used to assess the marrow’s involvement in some neoplastic conditions such as lymphoid neoplasia and mast cell neoplasia; to identify suspected infectious agents such as Histoplasma capsulatum, Leishmania spp., Ehrlichia spp., Cytauxzoon felis, and Toxoplasma gondii; and to evaluate patients with evidence of hyperglobulinemic conditions (e.g., multiple myeloma, lymphoma, ehrlichiosis or anaplasmosis, leishmaniasis, and disseminated histoplasmosis) (Box 27-1 and Box 27-2).7–11
Contraindications
Contraindications of bone marrow biopsies are few, and complications are uncommon. Restraint, sedation, and anesthesia usually pose a greater risk to the patient than the biopsy procedure. Bone marrow aspiration biopsies may often be performed without sedation; general anesthesia is seldom necessary. Hemorrhage is a theoretical concern in thrombocytopenic animals, but significant bleeding rarely occurs, even in severely thrombocytopenic animals. Bleeding complications may be controlled with fresh whole blood or appropriate component therapy.7,12,13 Iatrogenic marrow infection is possible, but the risk is miniscule, especially if the area of skin through which the aspirate is collected has been properly prepared. The main contraindication for bone marrow biopsy is performing an unnecessary biopsy. Evaluation of a current blood smear and a thorough clinical workup are mandatory before performing a bone marrow biopsy.
Sample Collection and Preparation
Proper collection and preparation of marrow are necessary for maximal diagnostic usefulness.7,8,10,14–16 Bone marrow degenerates rapidly after collection or the animal’s death; therefore, bone marrow samples from dead animals should be collected immediately (within 30 minutes) after the animal’s death. Regardless of whether the animal is alive or dead, bone marrow cytologic preparations should be prepared immediately after collection. Interestingly, granulocytes appear to be the first cells to undergo significant morphologic distortion after marrow sample collection or the animal’s death. Granulocytic nuclei swell and, losing their contorted lobulated pattern, become large and round to ovoid. The nuclear chromatin pattern loses its dense clumped areas and stains less intensely and more uniformly. As a result, neutrophils that have undergone this alteration may be mistaken for blast cells. This error may lead to misdiagnosis of neoplasia. Extreme caution must be used when examining samples from animals dead for 30 minutes or more before slide preparation. These samples may be examined for cellularity, organisms, mast cell infiltration, erythrophagocytosis, plasmacytosis, and accumulation of iron-containing pigments, but examination for evidence of neoplasia may result in misdiagnosis. The same requirements and limitations apply for bone marrow samples submitted for histopathologic examination.
Instruments and Supplies
A sterile 2% to 3% EDTA or isotonic saline solution may be prepared by using commercially available EDTA blood collection tubes, which contain about 1.5 milligrams (mg) of EDTA (as a freeze-dried powder or liquid solution) per milliliter of blood to be added. Injecting 0.35 mL of sterile isotonic saline into a 7-mL EDTA tube produces about 0.35 mL of 3% EDTA or 0.42 mL of 2.5% EDTA, depending on whether the tube originally contained powdered or liquid EDTA.
Aspiration Biopsy
Site
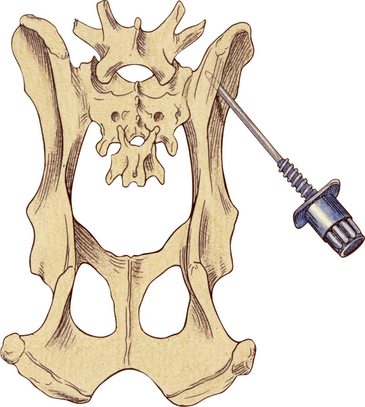
Bone marrow aspiration biopsies may be collected from the proximal humerus, iliac crest, trochanteric fossa of the femur, and sternebrae of dogs and cats (Figure 27-2, Figure 27-3, and Figure 27-4).7,8,10,14–16 The proximal humerus and iliac crest in large dogs and the proximal humerus and trochanteric fossa in small dogs and cats are the most accessible. As a result, they are the most commonly used sites. Because of the danger of penetrating the thoracic cavity, the sternebrae should be avoided in cats and small dogs. For the same reason, the ribs should be used only when incisional biopsies are performed. The trochanteric fossa may not be approachable in large, well-muscled, or very obese patients. Also, the cortical bone of the trochanteric fossa may be so dense in older dogs that it prevents easy penetration of the marrow cavity. Because of the small diameter of the bones of cats and small dogs, the trochanteric fossa often supersedes the iliac crest as a site for bone marrow aspiration in these animals.
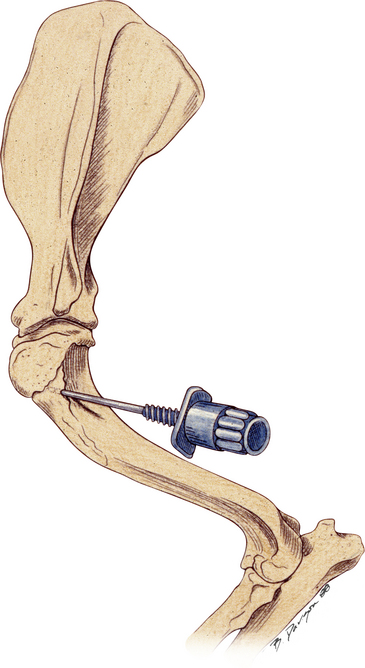
Figure 27-2 Bone marrow biopsy site for the proximal humerus.
This is a good site for both aspiration and core biopsies in small animals. (Reprinted with permission from Grindem CB: Bone marrow biopsy and evaluation, Vet Clin Small Anim 19[4]:673-4, 1989.)
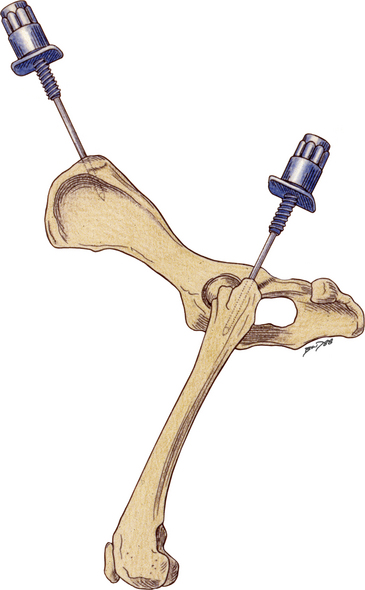
Figure 27-3 Bone marrow biopsy site for the iliac crest and proximal femur.
In large dogs, the dorsal approach to the iliac crest is an excellent site for aspiration and core biopsies. In small dogs and cats the lateral approach to the wing of the ilium is a good site for core biopsies. The trochanteric fossa of the proximal femur is a good site for aspiration biopsies. (Reprinted with permission from Grindem CB: Bone marrow biopsy and evaluation, Vet Clin Small Anim 19[4]:673-4, 1989).
Collection
To aspirate bone marrow via the humerus, trochanteric fossa of the femur, or the wing of the ilium, the animal is placed in lateral recumbency (see Figures 27-2, 27-3, and 27-4). If the iliac crest is used, the animal can be placed in the standing or sitting position or in sternal recumbency (see Figure 27-3). Regardless of the site used, the hair is clipped from skin several inches around the area where needle puncture is anticipated. Skin is then prepared as for surgery, and a local anesthetic is injected into skin and under the periosteum at the puncture site.
If marrow is not collected at the first site, the syringe is removed, the stylet is replaced in the biopsy needle, the needle is repositioned by slight advancement and rotation, and negative pressure is reapplied. If marrow is still not collected, the negative pressure is maintained and the needle is slowly withdrawn until marrow is obtained or the needle exits the bone. If this fails to collect an adequate sample, aspiration may be attempted from another site in the same bone or from another bone. If a sample cannot be collected by aspiration, a core biopsy or incisional biopsy is necessary.
Smear Preparation without EDTA
The sample is then immediately expelled directly onto a glass microscope slide. Direct smears similar to blood smears can be made and squash preps should also be prepared by tilting a slide 45 to 70 degrees, allowing the blood to drain from the slide into a watch glass or Petri dish (Figure 27-5). Marrow flecks tend to adhere to the glass microscope slide. A second glass microscope slide is placed perpendicularly across the marrow flecks adhered to the first slide, causing the marrow flecks to spread. The two slides are then smoothly pulled apart in a horizontal plane, dispersing the flecks. If the specimen clots, a piece of the clot is teased apart by the pusher slide, transferred to a clean glass slide, and squash preps prepared as described above.
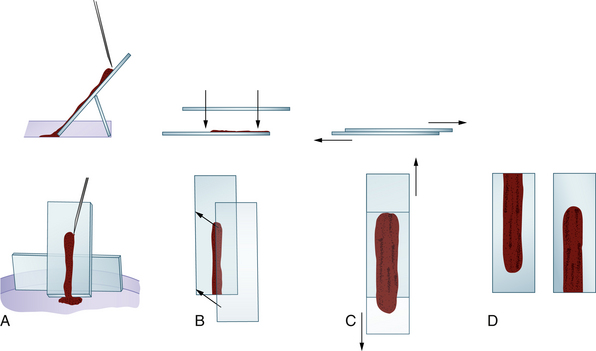
Figure 27-5 Smear preparation for bone marrow aspirates not collected in ethylenediaminetetraacetic acid (EDTA) or isotonic saline solution. A, Some of the aspirate is expelled onto a tilted glass microscope slide. The slide is propped up with another glass slide braced against the side of a Petri dish. Marrow flecks tend to adhere to the slide as the marrow runs down it. B, Another glass slide is placed over the slide containing the aspirate, which spreads the aspirate. C, The two slides are slid apart. D, This produces two preparations.
In Romanowsky-stained smears, the marrow flecks appear as blue-purple streaks. If the sample does not contain marrow flecks, the needle is repositioned by slight advancement and rotation. The stylet is removed, another syringe is attached, and the aspiration procedure is repeated. After two or three aspiration attempts or when marrow flecks are recovered, the needle is removed.
Smear Preparation with EDTA
Flecks are transferred from the sample in the Petri dish to glass microscope slides by tilting the Petri dish, causing the sample to drain to one side of the dish. Some flecks cling to the bottom of the Petri dish, and the fluid portion of the sample drains away from them. These flecks are harvested with a microhematocrit capillary tube, one end of which is touched to the side of the fleck. Often the fleck is partially aspirated into the capillary tube and is transferred to the glass microscope slide. If the fleck does not partially aspirate into the capillary tube, the tube is gently advanced, forcing the fleck into it. The marrow fleck is then transferred onto the glass microscope slide by tapping the capillary tube end containing the fleck on the slide. Any excessive fluid transferred is removed by touching the fluid with a piece of absorbent paper or cloth.
After the fleck is transferred to the glass microscope slide, a 22- × 22-millimeter (mm) coverslip is placed over the fleck at a 45-degree angle to the glass microscope slide, allowing one corner of the coverslip to hang over the edge of the microscope slide (Figure 27-6). When the coverslip is placed over the fleck, the fleck spreads to about twice its previous size. Some smears should be made without any pressure other than that caused by the coverslip, and others should be made with gentle thumb pressure sufficient to cause the smears to spread to about twice the diameter caused by coverslip pressure alone. This ensures that some of the flecks are spread optimally.
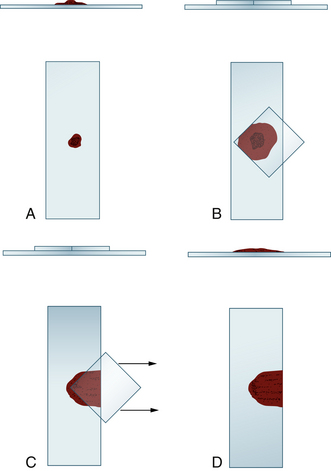
Figure 27-6 Smear preparation for bone marrow aspirates collected in ethylenediaminetetraacetic acid (EDTA) or isotonic saline solution.
A, A fleck, collected from the Petri dish containing the sample, is placed on a glass microscope slide. B, A microscope slide coverslip is placed over the fleck at a 45-degree angle to the slide. This spreads the fleck and accompanying fluid. C, The coverslip is slid horizontally and smoothly off the glass slide. D, Both the glass microscope slide preparation and coverslip preparation are used. However, the coverslip preparation is usually hard to handle during staining and is often discarded. Multiple preparations are made on a glass slide, and multiple slides are prepared.
Core Biopsy
Core biopsies are collected with a Jamshidi infant (cats and small dogs) or pediatric (large dogs) marrow biopsy needle. The same procedure, as described for collecting marrow aspirate biopsies, is used for collecting core biopsies; however, in this case, after the point of the needle has penetrated the cortex of the bone and entered the marrow cavity, the stylet is removed from the needle and the needle is advanced about 3 mm with a rotating motion.7,8,10,11,14–16 This cuts and collects a core of bone marrow. The needle is removed from the animal, and the core of marrow is forced onto a glass microscope slide by passing the stylet through the barrel of the needle from the hub end or retrograde from the needle end if the biopsy needle is tapered.
Using the point of the needle, the core of marrow is gently rolled the length of the microscope slide. After making one or two slide preparations in this manner, the core of marrow is placed in a container filled with 10% neutral buffered formalin.17 If the cytologic preparations are to be sent to an outside laboratory, they should not be mailed with the formalin-filled container because formalin vapors alter the cells’ staining qualities.
Collecting Marrow Samples From Dead Animals
Although samples are collected from most flat bones and the extremities of most long bones of dead animals, it is advisable to collect marrow samples from the same location(s) used in live animals (see Figures 27-2, 27-3, and 27-4). It should be kept in mind that in adults, the central areas of long bones contain mostly fat. Access to the marrow may be gained by sawing the bone, cutting it with rongeurs, or fracturing it. If the bone is sawed, heat generated at the saw line may damage adjacent cells; therefore, samples from sawed bones should be collected well away from the saw line. Marrow is dug from between bony trabeculae, trying to avoid collecting bone spicules. The marrow is placed on one end of a glass microscope slide and gently rolled (by lifting upward with a needle or other instrument at the back of the sample) the length of the slide. Several slides are prepared from samples collected from several different areas of the bone marrow. The slides are air-dried and stained as described later.
Cells In Marrow
To evaluate bone marrow preparations, one must be able to recognize the normal cells that occur in bone marrow, the neoplastic cells that have a propensity for infiltrating bone marrow, the organisms that can infect the marrow, and the processes (e.g., erythrophagia) that occur in some pathologic conditions.7–12, 16,18
Erythroid Series
During proliferation and maturation, erythroid progenitor cells undergo four to five mitoses, producing 16 to 32 daughter cells. As they mature, erythroid cells decrease in size, their nuclei condense, and their cytoplasm changes from dark blue to red-orange. The general characteristics of the different cell stages of erythroid production are described later. Table 27-1 lists the different stages of erythroid development and provides an estimate of the relative proportions and a brief description and a generalized schematic of the classic morphology of each stage.
TABLE 27-1
Nucleated Erythroid Cell Developmental Stages and Their Relative Percentage, Description, and Schematic Morphology
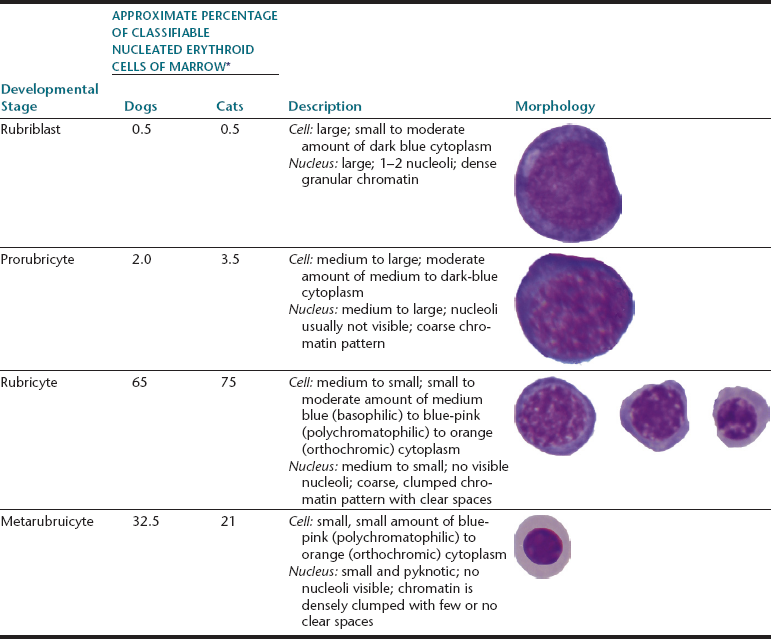
∗Broad variations may occur in normal animals because of the influence of things such as the subjectivity of cell classification and normal variation among individuals.
Rubriblasts
The rubriblast is the most immature identifiable erythroid cell. Its nucleus is round with a smooth nuclear border, a fine granular chromatin pattern, and one or two pale to medium blue nucleoli (Figure 27-7 and Figure 27-8). Its cytoplasm is intensely basophilic and forms a narrow rim around the nucleus. The rubriblast has the highest nucleus-to-cytoplasm (N:C) ratio of the erythroid series.
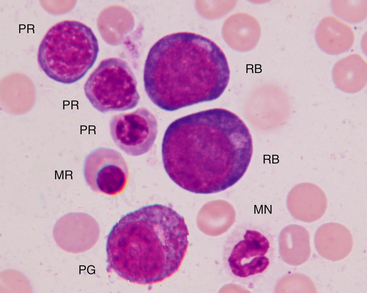
Figure 27-7 Bone marrow aspirate.
Rubriblasts (RB), polychromatophilic rubricytes (PR), metarubricyte (MR), progranulocyte (PG), and mature neutrophil (MN) are indicated. (Wright-Giemsa stain. Original magnification 250×.)
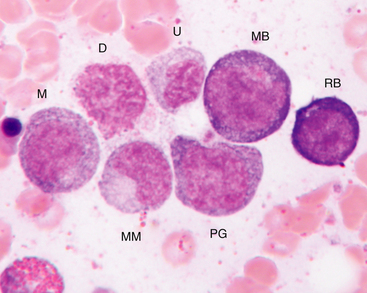
Figure 27-8 Bone marrow aspirate.
Rubriblast (RB), myeloblast (MB), progranulocyte (PG), early myelocyte (M), metamyelocyte (MM), damaged cell nucleus (D), and an unidentified late stage granulocyte (U), most likely distorted band neutrophil, are indicated. (Wright-Giemsa stain. Original magnification 250×.)
Rubricytes
The next stage of erythroid maturation is the rubricyte. This stage is divided into basophilic and polychromatophilic rubricytes or sometimes divided into basophilic, polychromatophilic, and orthochromic rubricytes. The rubricyte and its nucleus are smaller than the prorubricyte and its nucleus. The rubricyte nucleus has an extremely coarse chromatin pattern that may resemble the spokes of a wheel. Cytoplasm is blue (basophilic) to bluish-red-orange (polychromic or polychromatophilic) to red-orange (orthochromic) (Figure 27-9 and Figure 27-10; see Figure 27-7). The N:C ratio is less than that of prorubricytes but greater than that of metarubricytes (the next stage of maturation). Mitosis occurs in the early rubricyte stage but ceases by the later rubricyte stages.
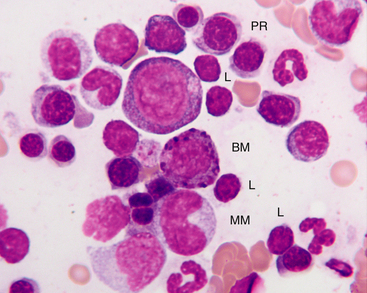
Figure 27-9 Bone marrow from a healthy cat.
Small lymphocytes (L) are usually less than 10% of nucleated cells in the bone marrow of healthy cats. Basophilic myelocyte (BM), neutrophilic metamyelocyte (MM), polychromatophilic rubricytes (PR), and lymphocytes are indicated. Basophil precursors have prominent magenta to blue-black granules. Also, note that metamyelocytes may be difficult to impossible to distinguish from monocytes in the bone marrow. (Wright-Giemsa stain. Original magnification 250×.)
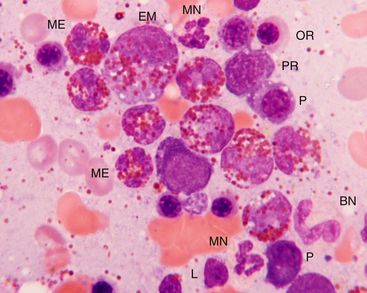
Figure 27-10 Bone marrow from a dog with peripheral eosinophilia (>50,000/µL).
The bone marrow was hypercellular with an increased myeloid-to-erythroid ratio, but eosinophils were the predominant granulocyte. Maturation appears orderly, but definitive cell stages may be difficult to determine because the eosinophilic granules cover the nucleus. Mature eosinophils (ME) outnumber earlier precursors such as eosinophilic myelocytes (EM). Mature neutrophils (MN), band neutrophils (BN), polychromatophilic rubricytes (PR), orthochromic rubricyte (OR), plasma cells (P), and a lymphocyte (L) are also present. Hypereosinophilic disorders include hypersensitivity reactions (often gastrointestinal), parasites, fungi, paraneoplasia, and very rarely eosinophilic leukemia. This dog was treated for heartworm disease, and the eosinophilia resolved. (Wright-Giemsa stain. Original magnification 250×.)
Metarubricytes
The next stage of erythroid development is the metarubricyte. Its nucleus is extremely pyknotic and appears black, without a distinguishable chromatin pattern (see Figure 27-7). Cytoplasm may be polychromatophilic or orthochromic.
Polychromatophilic Erythrocytes
The next stage of development is the polychromatophilic erythrocyte. In blood smears stained with Romanowsky-type stains, polychromatophilic erythrocytes are nonnucleated, larger than mature erythrocytes (orthochromic erythrocytes), and bluish-pink (polychromatophilic). Polychromatophilic erythrocytes stain as reticulocytes with supravital stains (e.g., new methylene blue [NMB]).
Histopathology of Erythroid Series
Erythroid precursors are often observed in erythropoietic islands surrounding a macrophage and located adjacent to sinuses (see Figure 27-1; Figure 27-11). It may be impossible to reliably distinguish rubriblasts from myeloblasts and to identify specific developmental stages, but the same general cytologic features of erythroid cells apply to histopathology. Erythroid series cells tend to be slightly smaller, have coarser chromatin patterns, have more prominent nucleoli, and the cytoplasm is more eosinophilic. The late stage erythroid precursors have small dark round almost pyknotic nuclei with a rim of eosinophilic cytoplasm. Small lymphocytes may look similar but lack the rim of cytoplasm (Figure 27-12 and Figure 27-13).
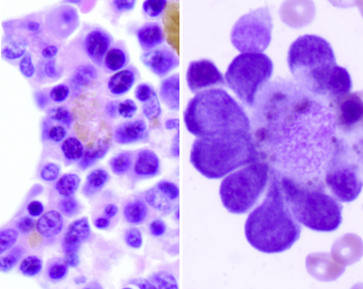
Figure 27-11 Bone marrow core biopsy from a dog (left).
Note the three erythropoietic islands with red blood cell precursors surrounding a central macrophage that contains brown-gold iron pigment. (H&E stain. Original magnification 400×.) The image on the right is the cytologic appearance of an erythropoietic island. (Wright-Giemsa stain (cytology). Original magnification 1000×.)
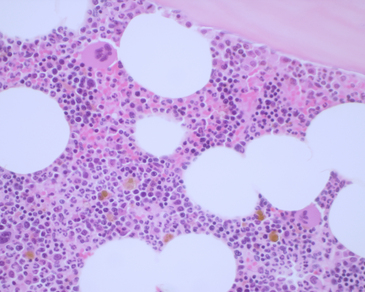
Figure 27-12 Hypercellular bone marrow from a young dog.
Note the trabecular bone at the top of the image, the few adipocytes (clear spaces), and the highly cellular hematopoietic tissue. The very dark areas represent active erythropoiesis, and the paler areas represent active myelopoiesis. The two very large cells are mature megakaryocytes. The golden brown pigment is iron. See Figure 27-13 for enlarged images. (H&E stain. Original magnification 400×.)
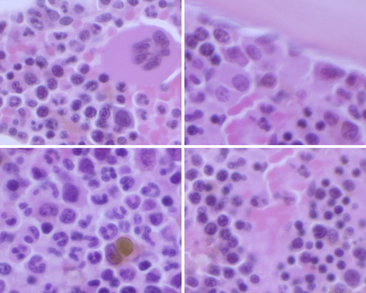
Figure 27-13 Enlargement of Figure 27-12.
Megakaryocytes and erythroid cells are often perivascular (upper left and lower right), whereas myeloblasts are paratrabecular (upper right) and maturing myeloid cells are interstitial (lower left). Note the erythropoietic islands in the upper left and lower right. Erythroid cells can be distinguished by their very dark condensing nuclei with scant cytoplasm. Maturing myeloid cells may be distinguished by their nuclear contour and lobulation. (H&E stain. Original magnification 400×.)
Granulocyte Series
Myeloid progenitor cells usually undergo four mitoses; however, depending on circumstances, a mitosis may be skipped or additional mitoses may occur. The developmental stages, immature to mature, of the granulocyte series are myeloblast, progranulocyte (promyelocyte), myelocyte, metamyelocyte, band cell, and segmented granulocyte (mature neutrophil, eosinophil, and basophil). The myeloblast, myelocyte, and metamyelocyte stages of neutrophilic granulocytes cannot be reliably differentiated from monocytes. Table 27-2 lists the different stages of development of the granulocyte series and gives the relative proportions and a brief description of the classic morphology of each stage.
TABLE 27-2
Developmental Stages of Neutrophils and Their Relative Percentage, Description, and Schematic Morphology
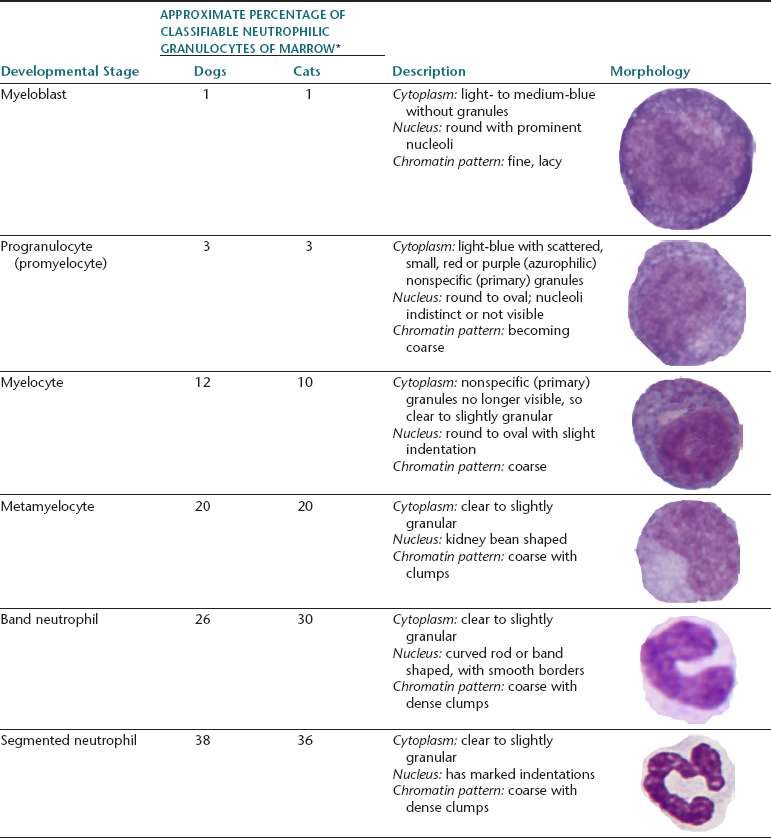
∗Broad variations may occur in normal animals because of the influence of things such as the subjectivity of cell classification and normal variation among individuals.
Myeloblasts
The myeloblast is large and may be round or irregular in shape. It has a large nucleus with a fine to finely stippled chromatin pattern and one or more visible nucleoli (see Figure 27-8). The cytoplasm is blue to blue-gray and does not contain visible granules. The myeloblast has the highest N:C ratio of any of the granulocytic developmental stages.
Progranulocytes (Promyelocytes)
The next stage of granulocyte development is the progranulocyte or promyelocyte. Often, the progranulocyte is slightly larger than the myeloblast because of increased cytoplasm. The major differentiating feature of the progranulocyte is the presence of scattered, small, azurophilic (red-purple) primary granules throughout the cytoplasm (Figure 27-14; see Figure 27-8). Primary granules are also called nonspecific granules. Nuclei are the same size to slightly smaller than nuclei of myeloblasts, have lacy to coarse chromatin patterns, and may contain visible nucleoli (which are usually fewer and less prominent than those of myeloblasts) or nucleolar rings. Nucleolar rings are rings of densely staining chromatin that demark obscured nucleoli. The N:C ratio of promyelocytes is less than that of myeloblasts. The presence of primary granules allows progranulocytes to be recognized as members of the granulocyte series and differentiates them from the monocyte series. Therefore, progranulocytes are identified more specifically than myeloblasts, neutrophil myelocytes, and neutrophil metamyelocytes, which lack visible primary granules. As a result, the term progranulocyte, instead of promyelocyte, is used to identify members of this developmental stage.
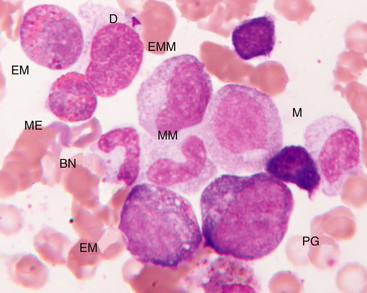
Figure 27-14 Bone marrow aspirate.
Progranulocyte (PG), neutrophilic myelocyte (M), eosinophilic myelocyte (EM), early metamyelocyte (EMM), metamyelocyte (MM), band neutrophil (BN), mature eosinophil (ME), and damaged cell nucleus (D) are indicated. (Wright-Giemsa stain. Original magnification 250×.)
Myelocytes
The next developmental stage of the granulocyte series is the myelocyte. The disappearance of the primary granules (seen in the progranulocyte stage) aids differentiation of the myelocyte from the progranulocyte. Also, myelocytes are smaller than progranulocytes, and their nuclear chromatin is more dense, giving a coarser pattern (see Figure 27-14; Figure 27-15; also see Figures 27-8 to 27-10). Nuclei are round to oval, and nucleoli are not visible. Cytoplasm is light blue to clear and contains secondary (specific) granules, which are poorly visualized in the neutrophil series but give eosinophils and basophils their characteristic appearance.
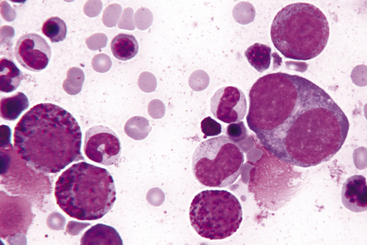
Figure 27-15 Bone marrow from a cat with eosinophilia and basophilia.
Note three basophilic myelocytes, a progranulocyte, and a binucleate promegakaryocyte. (Wright-Giemsa stain. Original magnification 250×.)
The granules of feline eosinophils are rod shaped, and the granules of canine eosinophils are pleomorphic (but typically round) and variable in size (see Figures 27-10 and 27-14). Vacuolated eosinophils (gray eosinophils) occur in Greyhounds and other sighthounds such as Italian Greyhounds and Whippets (Figure 27-16). Feline basophils contain granules that are oval, tend to stain lavender, and fill the cytoplasm. The oval granules may appear round when viewed end-on. Immature feline basophils contain many red-purple granules (see Figures 27-9 and 27-15). Canine basophils contain a few to many round, variably sized, red-purple (metachromatic) granules. The myelocyte is the last stage capable of mitosis.
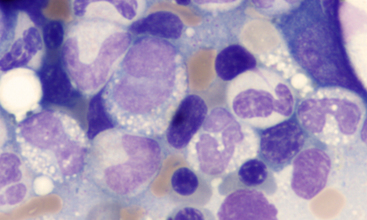
Figure 27-16 Bone marrow from a Greyhound dog.
Numerous vacuolated immature granulocytes (four cells in a zig-zag across the middle of the image) are mixed with cytologically unremarkable maturing neutrophil precursors. The vacuolated cells correlated with the peripheral eosinophilia (with gray eosinophils) in this dog. Gray eosinophils also occur in sighthounds other than Greyhounds, including Italian Greyhounds and Whippets. (Wright-Giemsa stain. Original magnification 1000×.)
Metamyelocytes
The next stage of granulocyte development is the metamyelocyte. Its nucleus is classically described as kidney bean shaped. Generally, cells with nuclei having indentations extending less than 25% of the way into the nucleus are classified as myelocytes, whereas those with nuclei having indentations extending 25% to 75% of the way into the nucleus are classified as metamyelocytes (see Figure 27-9). The cytoplasmic characteristics of metamyelocytes are similar to those of myelocytes, with the neutrophil myelocyte cytoplasm being clear. This stage and subsequent stages do not undergo mitosis.
Band Cells
The next stage of granulocyte development is the band cell. It has a nucleus with a curved band or rod shape and smooth, parallel sides (see Figures 27-10 and 27-14). Some chromatin clumps are present. No area of the nucleus has a diameter less than half the diameter of any other area of the nucleus. Membrane irregularity and excessive narrowing of the nucleus warrant classifying of the cell as a mature neutrophil. The cytoplasmic characteristics of band cells are similar to those of metamyelocytes.
Segmented Granulocytes
The final stage of granulocyte development is the segmented granulocyte (mature neutrophil, eosinophil, or basophil). Its nucleus is lobate or has areas of marked constriction (see Figure 27-10). The nuclear border is often irregular with large, dense chromatin clumps. Its cytoplasmic characteristics are similar to those of myelocytes, metamyelocytes, and band cells.
Monocyte Series
Histopathology of Myeloid Series
Myeloblasts and promyelocytes are frequently found adjacent to trabecular bone and should not be mistaken for neoplastic cells (see Figure 27-12 and 27-13). These cells have large round to oval nuclei, sparse chromatin, prominent nucleoli, and scant pale cytoplasm. Later-developing granulocytes are more dispersed in the interstitial areas of the marrow cavity. Metamyelocytes, bands and segmented granulocytes are identified by their nuclear indentations. In a normal bone marrow, approximately 80% to 85% of the myeloid cells should be metamyelocytes, bands, and segmented granulocytes. Eosinophils are distinguished by their distinctive granules, whereas basophils and monocytes cannot be reliably identified.
Thrombocyte (Megakaryocyte) Series
Promegakaryocytes
The promegakaryocyte is the next recognizable stage. It has deep blue cytoplasm and contains two to four nuclei that may appear separate but are linked by thin strands of nuclear material (see Figure 27-15). Promegakaryocytes are much larger than white blood cell (WBC) and red blood cell (RBC) precursors.
Megakaryocytes
The megakaryocyte is the next developmental stage. Megakaryocytes are gigantic (50 to 200 micrometers µm] in diameter) and contain more than four nuclei that are joined and form a lobulated mass (Figure 27-17 and Figure 27-18). The larger the cell, the greater is the nuclear ploidy (number). Megakaryocytes with deeply basophilic cytoplasm are less mature than those with light blue cytoplasm containing eosinophilic granules. Immature megakaryocytes are sometimes called basophilic megakaryocytes, and mature megakaryocytes are sometimes called eosinophilic or granular megakaryocytes. Bare nuclei of megakaryocytes may be seen in bone marrow cytologic preparations. They may represent nuclei of megakaryocytes that have shed their cytoplasm as platelets into the peripheral blood or megakaryocytes whose cytoplasm has been torn from their nuclei during smear preparation.
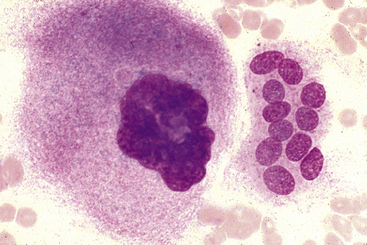
Figure 27-17 Bone marrow aspirate.
Mature megakaryocyte with a condensed multilobulated nucleus and eosinophilic, granular cytoplasm on the left and a multinucleated osteoclast on the right. Osteoclasts are uncommonly seen in bone marrow aspirates and are associated with bone remodeling such as in young animals or animals with renal disease, metabolic bone disease, or neoplasia. (Wright-Giemsa stain. Original magnification 240×). (Reprinted with permission from Grindem CB: Bone marrow biopsy and evaluation, Vet Clin Small Anim 19[4],680, 1989.)
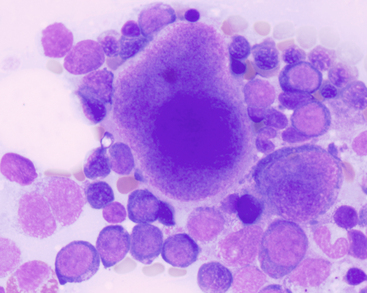
Figure 27-18 Bone marrow aspirate from a dog with megakaryocyte hyperplasia.
A promegakaryocyte with two nuclei is in the lower left, an almost mature megakaryocyte with eosinophilic cytoplasm is in the center, and an immature basophilic megakaryocyte is on the right. Note the concurrent erythroid hyperplasia with left shifting (more immature precursors). (Wright-Giemsa stain. Original magnification 1000×.)
Histopathology of Megakaryocyte Series
Megakaryocytes are the largest cells encountered in the bone marrow (see Figure 27-12 and 27-13). Mature megakaryocytes have more condensed chromatin pattern and more abundant eosinophilic cytoplasm than immature megakaryocytes. Megakaryoblasts are difficult to distinguish from other blast cells. Megakaryocytes appear randomly distributed within the interstitium but, in fact, are adjacent to inconspicuous sinusoids. Generally two to four megakaryocytes per high-power magnification (40×) is regarded as adequate.1 Since osteoclasts are another large cell identified in histologic sections, it is important to distinguish between these and megakaryocytes. Megakaryocytes will have a multilobulated nucleus, rather than the multiple small distinct nuclei in osteoclasts, and megakaryocytes will often be distributed in an interstitial or peri-sinusoidal location, rather than in the paratrabecular location of osteoclasts. Also, multinucleate inflammatory, or neoplastic giant cells may be differentiated from megakaryocytes by the “company they keep.” These multinucleate cells will be associated with other inflammatory cells or neoplastic cells, rather than with hematopoietic cells.
Lymphocytes and Plasma Cells
Small lymphocytes (Figure 27-19; see Figure 27-9) are recognizably smaller than neutrophils and have a scant amount of light- to medium-blue cytoplasm and an indented but otherwise round nucleus with a dense smudged chromatin pattern without visible nucleoli. Intermediate-sized lymphocytes (often referred to as prolymphocytes) are about the same size as neutrophils and have a little more medium to light-blue cytoplasm compared with small lymphocytes. Their nuclei are round, other than an indentation in one area of the nucleus where the cytoplasm is most visible. The nuclear chromatin pattern appears smudged and nucleoli are rarely visible. Large lymphocytes, frequently called lymphoblasts when their nucleoli are prominent (Figure 27-20), are larger than neutrophils and have a small to moderate amount of light- to dark-blue cytoplasm. Their nuclei may be indented or irregular and have a fine reticular or lacy chromatin pattern. Multiple prominent nucleoli often are present.
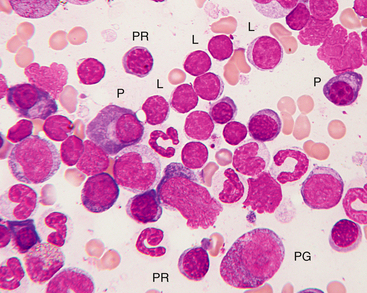
Figure 27-19 Bone marrow from a healthy cat.
A focal area of numerous small lymphocytes is present. Small lymphocytes (L) may easily be confused with nucleated red blood cells, but small lymphocytes have scant to almost no visible cytoplasm and often a less coarsely clumped nuclear chromatin pattern. Small lymphocytes usually comprise less than 5% of nucleated bone marrow cells in healthy dogs and less than 10% in cats. Progranulocyte (PG), polychromatophilic rubricytes (PR), plasma cell (P), and lymphocytes are indicated. (Wright-Giemsa stain. Original magnification 250×.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree