T
Thoracocentesis
INDICATIONS
• Animals with respiratory distress (increased respiratory rate and effort) and dull lung sounds on auscultation
• Animals that present after trauma (hit by car, bite wounds, falling from height) and those that have undergone positive-pressure ventilation have increased risk for pneumothorax and may require thoracocentesis
EQUIPMENT, ANESTHESIA
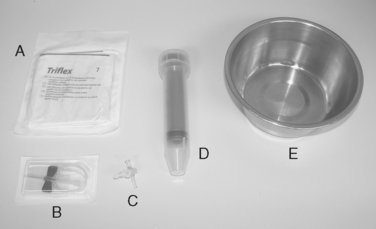 THORACOCENTESIS Materials used for thoracocentesis for a cat or small dog. A, Sterile gloves. B, Butterfly-type catheter. C, Three-way stopcock. D, Large syringe. E, Bowl.
THORACOCENTESIS Materials used for thoracocentesis for a cat or small dog. A, Sterile gloves. B, Butterfly-type catheter. C, Three-way stopcock. D, Large syringe. E, Bowl.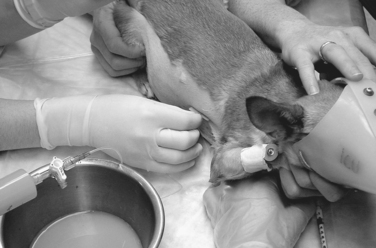 THORACOCENTESIS Thoracocentesis for removal of septic pleural effusion from a Chihuahua. A butterfly catheter with three-way stopcock and a 60-mL syringe are in use. The entry site is at the right seventh or eighth intercostal space, approximately at the level of the costochondral junction. An open muzzle is placed loosely for protection of staff without compromising the animal’s respirations.
THORACOCENTESIS Thoracocentesis for removal of septic pleural effusion from a Chihuahua. A butterfly catheter with three-way stopcock and a 60-mL syringe are in use. The entry site is at the right seventh or eighth intercostal space, approximately at the level of the costochondral junction. An open muzzle is placed loosely for protection of staff without compromising the animal’s respirations.PREPARATION: IMPORTANT CHECKPOINTS
• If the patient is in severe respiratory distress and has dull lung sounds on auscultation, thoracocentesis should be performed prior to thoracic radiographs.
• In cases where respiratory signs are less severe, thoracic radiographs can be used for confirming the presence of fluid or air in the pleural space prior to thoracocentesis.
• Rule out coagulopathy as likely cause of respiratory signs first (from history, physical examination, ± coagulation screening).
PROCEDURE
• Position animal, preferably in sternal recumbency or standing, but lateral recumbency is also acceptable for pneumothorax.
• Have assistant available to restrain animal or give sedation as needed:
○ Consider brief, quiet rest in oxygen cage if patient is anxious and extremely dyspneic (any restraint or sedation appears hazardous).
• Clip and aseptically prepare appropriate rib space:
○ If expecting fluid or if unsure (fluid versus air), clip at the seventh or eighth intercostal space (ICS), about at the level of the costochondral junction.
• Insert needle slowly, bevel side up, just cranial to the rib to avoid intercostal blood vessels. When through skin (beveled edge of needle is no longer visible), begin aspirating with a few tenths of 1 mL of negative pressure for a cat or small dog to 1-2 mL of negative pressure for larger patients, respectively.
• Observe hub of needle for signs of fluid (“flashback”):
○ If a small amount of frank blood is aspirated or if lungs can be felt rubbing against needle, needle should be moved to a different location.
• Directing the needle ventrally, rolling the animal slightly to the side on which thoracocentesis is being performed, and reaspirating from a more ventral location can facilitate removal of as much fluid as possible.
• Ultrasound guidance can be beneficial in finding small fluid pockets for diagnostic thoracocentesis.
• Fluid is submitted for fluid analysis and cytologic examination and is saved for future bacterial culture and sensitivity (C&S) (aerobic, anaerobic) if cytologic examination suggests septic exudate.
ALTERNATIVES AND THEIR RELATIVE MERITS
• Chest tube placement: continuous removal of fluid and air but is more invasive and is associated with a greater risk of iatrogenic complications.
• Diuretics: slow mobilization of modified transudates (e.g., heart failure) compared to thoracocentesis and ineffective with other causes (e.g., exudates, hemorrhage).
Thoracoscopy
OVERVIEW AND GOAL
• A procedure for endoscopic exploration, biopsy and surgical procedures of the thorax (pericardial window, vascular ring surgery, patient ductus arteriosus ligation, thoracic duct ligation, lung lobectomy)
• Thoracoscopy is performed almost exclusively on dogs; size limitations limit visualization and safe manipulation in patients < 7 kg.
INDICATIONS
• Idiopathic pneumothorax: rarely done, may be done as adjunctive step for surgical planning for thoracotomy
EQUIPMENT, ANESTHESIA
• Endoscopic instruments. For basic pericardial window or pleural biopsy: 360○ rotatable Metzenbaum scissors, Babcock atraumatic grasping forceps, cup biopsy instrument
• Premedication with opioid (e.g., hydromorphone, 0.1-0.2 mg/kg IM or IV) and benzodiazepine (e.g., midazolam, 0.2-0.4 mg/kg IV or IM); anticholinergic agents administered only as needed.
PREPARATION: IMPORTANT CHECKPOINTS
• The entire ventral and lateral thorax and abdomen should be clipped and aseptically prepared to facilitate quick conversion to an open thoracotomy or laparotomy in an emergency setting.
POSSIBLE COMPLICATIONS AND COMMON ERRORS TO AVOID
• Insufficient procedural analgesia will result in “bucking” of the ventilator (voluntary breaths, not coordinated with mechanical ventilation). This results in rapid oxygen desaturation.
PROCEDURE
• A Duke’s trocar is used for inducing pneumothorax and to allow the threading of a 12 Fr suction tube into the left approximately 10th intercostal space (ICS) just dorsal to the mid-left thorax. After placing a three-way stopcock, additional room air is introduced into the thorax (≈10-20 mL/kg) to create a space in which to work, but being mindful of excess (e.g., overinsufflation causing inadequate oxygen saturation/hypoxemia).
• The long (=15 cm) trocar is introduced through an approximately 8-mm stab incision through the skin and subcutaneous tissues approximately 5 cm caudal and to the left of the xiphoid process. The trocar is introduced at a downward angle (i.e., craniodorsally) and then positioned parallel to the abdominal body wall and aimed toward the left shoulder joint. The trocar is removed and the endoscope placed through the trocar when the sensation of crossing the diaphragm is felt or at any point to monitor progress. The trocar is almost fully inserted when traversing the diaphragm of large dogs.
• Two additional instrument ports are placed on the left hemithorax in a triangulated orientation: approximately at the eighth ICS 5-10 cm below (dorsal to) the costochondral junction, and the seventh ICS 2-5 cm below the costochondral junction. Exact location depends on chest conformation and heart size. Prior to introduction of the trocars, the selected intercostal locations are palpated and visualized internally with the endoscope to decide if they are suitable, considering heart and lesion location. If so, an 8-cm stab wound is made into the skin to allow placement of a 5-mm endoscopic trocar with a closed valve.
• With ports created and instruments in place, the heart is visualized, and the forceps (in the cranial port) and scissors (in the caudal port) are visualized within the thorax. The pericardium is grasped. This may be difficult in patients with pericardial effusion at the time of the procedure or in patients with a very thick pericardial sac. The pericardial sac must be grasped—not just the fatty layer adjacent to it—to allow for adequate manipulation. One clue is the apex beat of the heart that can be felt on the forceps when the pericardium, but not pericardial fat, is contacted.
• When the pericardium is grasped, a small incision should be made into it, close to the tips of the forceps and with the pericardium tented up and away from the underlying heart. Care should be taken to not inadvertently clip any pulmonary tissue. Upon successful entry of the pericardium, fluid will be released in patients with pericardial effusion. The pericardium can then be repositioned with one jaw of the forceps within the pericardial sac for better control. The goal with a pericardial window is to make an opening approximately 4-5 cm in diameter for a large-breed dog. This will result from a small incision in a normal elastic pericardium, or a larger amount of tissue removed in multiple pieces when the pericardium is diseased and more rigid. The pericardial window should be made on the sternal surface of the heart to avoid the phrenic nerves coursing craniocaudally along the dorsal third of the pericardium.
• After the pericardial window is completed, the pericardium is tented to attempt a brief look inside the pericardial sac for mass lesions at the heart base. Tumors may be visualized in this position occasionally, but care should be taken to not induce arrhythmias or cause tumor bleeding. Tumor biopsy may be considered.
• The instrument ports are removed, using internal visualization of the sites for hemorrhage suggesting transection of an intercostal artery. Once adequate hemostasis is confirmed, the camera and transabdominal port are removed.
• The incisions are closed with a single cruciate suture in the subcutis (3-0 absorbable) and a single staple or cruciate suture in the skin. The chest tube is anchored with a modified Chinese-finger-trap suture (2-0 nonabsorbable suture). The air and any fluid are evacuated from the thorax. For local analgesia, bupivacaine (0.2 mL/kg) is diluted 1 : 1 with saline and placed into the thorax through the chest tube, with the patient in left lateral recumbency. The chest tube is additionally secured with a light chest wrap.
• Postoperatively, hydromorphone is administered for a minimum of two doses as dictated by patient recovery.
• A short, 5-cm diameter endoscopic trocar is introduced in the approximately ninth intercostal space just dorsal to the costochondral junction. The camera is introduced and facilitates preliminary exploration of the thorax.
• A second trocar is introduced approximately in the fourth to fifth intercostal space just dorsal to the costochondral junction. Care is taken to introduce the trocar just through the chest wall, and then remove the sharp inner cannula to further introduce only the blunt outer cannula. The camera is positioned carefully across the mediastinum to the right hemithorax.
• A biopsy instrument is manipulated over (ventral to) the heart and toward the field of vision of the endoscope, using the transillumination of the skin as a guide to facilitate biopsy selection of the pleura. In patients with a normal-appearing parietal pleura, attempts are made to select biopsy samples from the diaphragmatic pleural reflection, which may be a place where occult mesothelioma cells may be easier to find.
• If the disease process appears lateralized, the procedure may be repeated on the other side of the thorax (from the right side to biopsy the left hemithorax).
POSTPROCEDURE
• Repeat chest tube aspirations to collect residual effusions/air and to monitor for bleeding complications.
• Nonsteroidal antiinflammatory drug (NSAID) generally started the first postoperative day after patient starts eating (unless contraindicated) and continued for 2-3 days (e.g., carprofen, 1 mg/kg PO q 12 h).
• Chest tube is removed 6-12 hours post procedure unless there are large amounts of ongoing fluid or air drainage.
ALTERNATIVES AND THEIR RELATIVE MERITS
For recurrent pericardial effusion:
• Thoracotomy:
○ Advantage: complete thoracic exploration, easier hemorrhage control, and facilitates full subtotal pericardectomy in patients with restrictive pericardial disease. Less specialized equipment required.
Tracheal Stent Placement
OVERVIEW AND GOAL
• Tracheal stent placement is most often utilized for the management of tracheal collapse in dogs. Tracheal stents may also be utilized for the management of tracheal neoplasia or other causes of tracheal obstruction. The following information will describe the technique for tracheal stent placement for the management of tracheal collapse.
• Conservative management of tracheal collapse is centered on medical management including cough suppressants, corticosteroids, and management of concurrent problems including but not limited to pneumonia, bronchitis, obesity, and heart disease.
• When conservative management cannot adequately control the clinical signs, medications are poorly tolerated, or quality of life is compromised, palliative interventions including surgery for prosthetic ring placement or image-guided tracheal stent placement must be considered. It is critical for the client to accept that these interventions are palliative and will not cure the problem. Instead, the goal of these procedures is a significant improvement in clinical signs and less reliance on medical therapies.
INDICATIONS
• Tracheal collapse no longer amenable to conservative therapy as described above, involving extrathoracic and/or intrathoracic segments of the trachea
• Emergency management of dogs presented in crisis due to tracheal collapse that cannot be extubated due to recurrent airway obstruction
EQUIPMENT, ANESTHESIA
• Fluoroscopic guidance is most often utilized for tracheal stent placement. Digital radiography systems have been employed but lack the “real-time” image acquisition that allows for optimal stent placement. Bronchoscopic guidance has been described.
• General anesthesia is required for tracheal stent placement. Most often, a premedication that includes an opioid for its antitussive effects is utilized. Propofol induction and inhalant anesthetic in oxygen are appropriate in most patients undergoing tracheal stent placement.
• Bronchoscope adapter allows for maintenance of the anesthetic circuit during tracheal stent deployment.
• Inventory of various lengths and diameters of self-expanding metallic stents. Most commonly utilized brands include the Vet-Stent Trachea (Infiniti Medical LLC, Malibu, Calif.) and the Wallstent or Ultraflex (Boston Scientific, Natick, Mass.). A complete inventory allows the veterinarian to transition smoothly from diagnosis to treatment during a single anesthetic event.
• Shortening charts for the brand of stent utilized. These charts provide information about stent length when the stent does not open to its nominal diameter.
• Standardized marking device (marker catheter) placed at the level of the trachea during imaging to allow for accurate measurements of the tracheal dimensions such that the effects of magnification can be minimized. The marker catheter has radiopaque markings that are spaced 1 cm apart.
PREPARATION: IMPORTANT CHECKPOINTS
• Perform fluoroscopy on the patient with tracheal collapse prior to anesthesia induction to determine the length of the airway obstruction relative to anatomic landmarks. Note the presence of concurrent mainstem bronchial collapse.
• Perform a laryngeal exam at anesthetic induction. If structure or function is abnormal, these problems will require management.
• Perform tracheobronchoscopy to assess the severity of tracheal and mainstem bronchial collapse. Contrast with fluoroscopic assessment to definitively determine the location and severity of the tracheal collapse.
POSSIBLE COMPLICATIONS AND COMMON ERRORS TO AVOID
• Acute complications of tracheal stent placement are very rare when the technique is performed appropriately.
• Without a marker catheter or similar measurement standardization device placed at the level of the trachea, magnification could significantly (and adversely) affect measurements.
• Undersizing of the tracheal stent may occur if the trachea is not appropriately distended with positive pressure (20 cm H2O) during measurement. Undersizing results in stent migration.
• Foreshortening is a property of many woven self-expanding metallic stents. When constrained on the delivery system, the stent will appear longer than its nominal (relaxed) diameter. As the stent is deployed, it will shorten towards its nominal length. Conversely, if the stent does not fully expand within the trachea (as expected owing to oversizing), it will be longer than the nominal length. Failure to recognize the properties of the stent can result in choosing a stent longer or shorter than is needed.
• Tracheal stents may be accidentally deployed into the carina, endotracheal tube, or larynx if inappropriately sized for the length of the tracheal collapse.
PROCEDURE
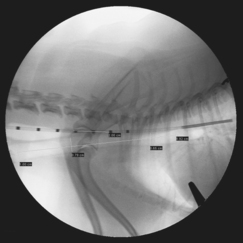
• Anesthetist is asked to hold a positive pressure breath at 20 cm H2O in the anesthetic circuit while a radiograph is acquired. This maneuver is critical to determine the maximal tracheal diameter. Measurements of the tracheal diameter are then performed in the cervical and intrathoracic trachea.
• The stent diameter should be chosen to exceed the maximal tracheal diameter (usually a cervical measurement) by 10%-20%. As an example, if the cervical trachea measures 10 mm and the thoracic trachea measures 8 mm, then a 12-mm stent is chosen (10%-20% larger than the maximal tracheal diameter).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree