Chapter 12
Synovial Fluid Analysis
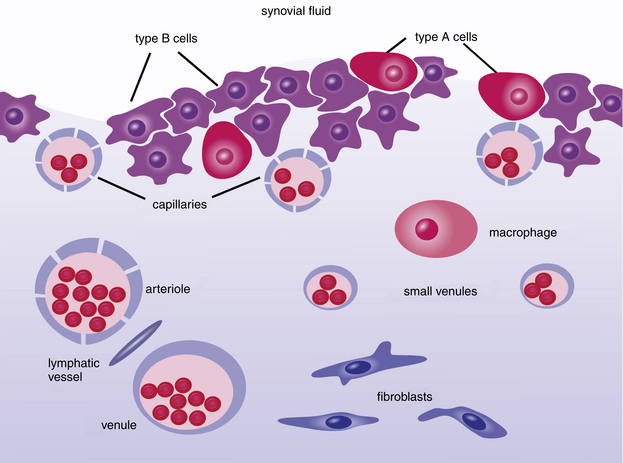
As fluid enters and leaves the joint cavity, its diffusion and composition is regulated by connective tissue of the subintima and cells of the intima, or synovial lining. The intima is made-up mostly of secretory, fibroblast-related, synoviocytes (type B cell) and fewer macrophages (type A cell). The type B cells, which constitute 70% to 90% of intimal cells, secrete components for tissue interstitium and synovial fluid that include collagens, fibronectin, hyaluronan, and lubricin.1,2 Type A are derived from bloodborne mononuclear cells and are considered resident tissue macrophages, much like hepatic Kupffer cells (Figure 12-1 and Figure 12-2). Type B cells and type A cells demonstrate immunohistochemical reactivity to heat shock protein 25 (HSP25) and CD18, respectively.1
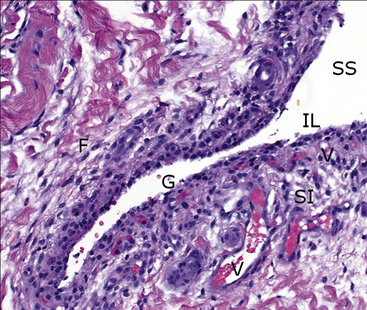
Figure 12-2 Histologic specimen of synovial membrane showing details within the valley of a normal fold in the lining.
Directly interfacing with the synovial space (SS) is a sparse intimal layer (IL), only one to two cells thick, with underlying vessels (V) embedded among fibrous subintima (SI). Note the normal acellular gap in the intimal lining (G) and fibrocytes (F) within the subintima. (H&E stain. original magnification 20×.) (Courtesy of Dr. Dave Getzy.)
Arthrocentesis
In the verification, localization, diagnosis, and management of arthritis, synovial fluid examination is a key component of an initial medical database that includes clinical history, physical examination, radiographs, complete blood count, biochemical profile, and urinalysis. See Box 12-1 and Box 12-2 for indications and contraindications for arthrocentesis.
Equipment
Sterile disposable 3-milliliter (mL) syringes and 1-inch, 22-gauge or 25-gauge (for small dogs and cats), hypodermic needle are recommended. In large-breed dogs, sampling of the elbow or shoulder joints may require a 1½-inch needle and the hip joint may necessitate a 3-inch spinal needle. Microscope glass slides with frosted ends, red-top tubes, and ethylenetetraacetic acid (EDTA) blood tubes should be readied and labeled with the patient’s name and the joint sampled. See Box 12-3 for a complete list of materials.
Approaches
Carpal Joint
Entry is obtained via the antebrachiocarpal joint or the middle carpal joint. In either case, the carpus is flexed to increase access to the joint’s spaces. The needle is introduced from the dorsal aspect, just medial of center, then inserted perpendicular to the joint. Landmarks for the antebrachiocarpal joint are the distal radius and the proximal radial carpal bone (Figure 12-3). The middle carpal joint is between the distal portion of the radial carpal bone and the second and third carpal bones.
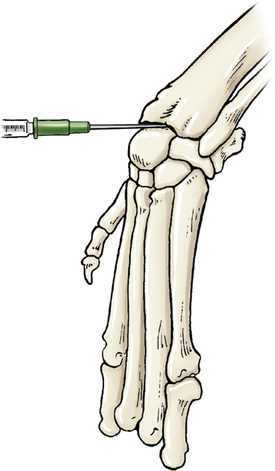
Figure 12-3 Arthrocentesis of the carpus joint.
The joint may be located by applying fingertip pressure just distal to the radius during flexion and extension. The needle is introduced between the distal radius and proximal to the radial carpal bone. (From Piermattei DL, Flo G, DeCamp C: Chapter 1 – Orthopedic Examination and Diagnostic Tools. In: Brinker, Piermattei, and Flo’s Handbook of small animal orthopedics and fracture repair, ed 4, St. Louis, MO, 2006, Saunders [p. 24].)
Elbow Joint
Entry to the elbow may be attained with the joint in extension or flexion. Hyperextension of the elbow allows the needle to be introduced medial to the lateral epicondyle of the humerus and lateral to the olecranon. Once in the joint space the needle is guided cranially toward the humeral condyle (Figure 12-4). With the elbow in a 90-degree angle of flexion, the needle can be introduced just proximal to the olecranon and medial to the lateral epicondylar crest. The needle will be inserted parallel to the olecranon and the long axis of the ulna.
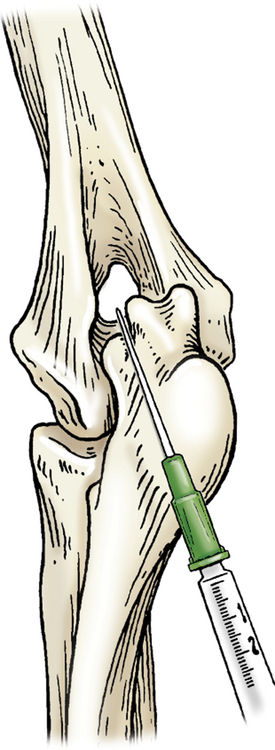
Figure 12-4 Arthrocentesis of the elbow joint.
With the elbow in hyperextension, the needle is introduced medial to the lateral epicondyle of the humerus and lateral to the olecranon. (From Piermattei DL, Flo G, DeCamp C: Chapter 1 – Orthopedic Examination and Diagnostic Tools. In: Brinker, Piermattei, and Flo’s Handbook of small animal orthopedics and fracture repair, ed 4, St. Louis, MO, 2006, Saunders [p. 23].)
Shoulder Joint
Access is gained from the lateral aspect, with the needle introduced distal to the acromion of the scapula and caudal to the greater tubercle of the humerus. The needle is directed medially toward the greater tubercle and distal to the supraglenoid tubercle of the scapula (Figure 12-5).
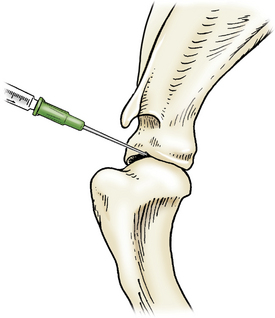
Figure 12-5 Arthrocentesis of the shoulder joint.
The needle is introduced distal to the acromion of the scapula and caudal to the greater tubercle of the humerus and then directed medially towards the greater tubercle and just distal to the supraglenoid tubercle of the scapula. (From Piermattei DL, Flo G, DeCamp C: Chapter 1 – Orthopedic Examination and Diagnostic Tools. In: Brinker, Piermattei, and Flo’s Handbook of small animal orthopedics and fracture repair, ed 4, St. Louis, MO, 2006, Saunders [p. 23].)
Tarsal Joint
Access is gained via a cranial or caudal approach. In the cranial approach, the tarsus is slightly flexed, and the needle is introduced at the space palpated between the tibia and talus (tibiotarsal) bones, just lateral to the tendon bundle. For the caudal approach, the joint is extended and the needle can be inserted medial or lateral to the calcaneus (fibular tarsal bone) with a cranial and slightly plantar path (Figure 12-6).
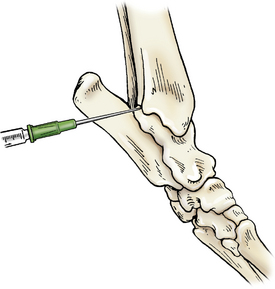
Figure 12-6 Arthrocentesis of the tarsal joint.
The joint is extended and the needle is inserted medial to the calcaneus in a cranial path. (From Piermattei DL, Flo G, DeCamp C: Chapter 1 – Orthopedic Examination and Diagnostic Tools. In: Brinker, Piermattei, and Flo’s Handbook of small animal orthopedics and fracture repair, ed 4, St. Louis, MO, 2006, Saunders [p. 22].)
Stifle Joint
The stifle is flexed, and the needle is introduced just lateral to the patellar ligament and distal to the patella. The needle is advanced in a medial and proximal direction pointing toward the medial condyle of the femur (Figure 12-7).
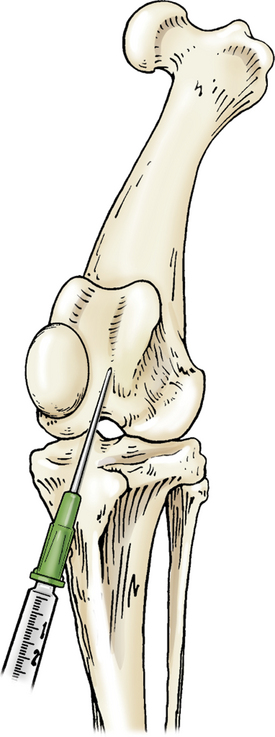
Figure 12-7 Arthrocentesis of the stifle joint.
The stifle is flexed and the needle introduced lateral to the patellar ligament and distal to the patella and advanced in a medial and proximal direction toward the medial condyle of the femur. (From Piermattei DL, Flo G, DeCamp C: Chapter 1 – Orthopedic Examination and Diagnostic Tools. In: Brinker, Piermattei, and Flo’s Handbook of small animal orthopedics and fracture repair, ed 4, St. Louis, MO, 2006, Saunders [p. 22].)
Hip Joint
The femur is abducted and the leg extended caudally. The needle is introduced cranial to the greater trochanter of the femur and inserted caudal and distal or ventral toward the joint (Figure 12-8).
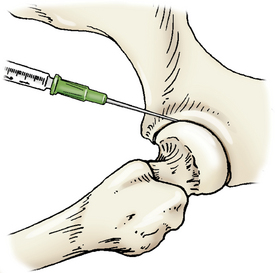
Figure 12-8 Arthrocentesis of the hip joint.
The femur is abducted with the needle introduced cranial to the greater trochanter of the femur and guided caudal and distal toward the joint. (From Piermattei DL, Flo G, DeCamp C: Chapter 1 – Orthopedic Examination and Diagnostic Tools. In: Brinker, Piermattei, and Flo’s Handbook of small animal orthopedics and fracture repair, ed 4, St. Louis, MO, 2006, Saunders [p. 21].)
Sample Handling and Test Priorities
Laboratory tests performed may be limited by volume of synovial fluid collected. While the sample is in the syringe, volume, color, and turbidity should be noted. Viscosity is then assessed as the sample is expelled onto a glass slide for direct smears. Direct smears are immediately made for subsequent cytologic examination, nucleated cell differential count, and subjective assessment of cellularity. See Table 12-1 and Table 12-2 for specific volumes needed and sequence of testing. When larger volumes of fluid are collected, a total nucleated cell count, mucin clot test, and total protein estimation, in order of priority, may be added to the aforementioned procedures.
TABLE 12-1
TEST PRIORITIES FOR 2 ML OR MORE SYNOVIAL FLUID
| Amount | Test | |
| 1 drop | Cytology and white blood cell differential with viscosity estimate | Glass slide |
| 0.5 to 1.0 mL | Total nucleated cell count | Lavender top or plain |
| 20-mL (pediatric) Becton Dickinson BBL™Septi-Chek™ blood culture tube | ||
| Or | Or | Or |
| 0.5 to 1.0 mL | Bacterial culture and sensitivity | Sterile plain blood tube |
TABLE 12-2
TEST PRIORITIES FOR LESS THAN 1 ML OF SYNOVIAL FLUID
| Amount | Test | |
| 1 drop | Cytology and white blood cell differential with total nucleated cell estimate and viscosity | Glass slide |
| 2 or 3 drops | Bacterial culture and sensitivity | Culturette |
Cells in sediment smears and direct smears of fluid with the normally high viscosity may not spread out well on slides, making cell identification and differential cell count difficult. If this problem is encountered, it may be overcome by mixing an equal volume of hyaluronidase at 150 international units per millilter (IU/mL) with the synovial fluid and incubating for at least 10 minutes.3 The result is a fluid that facilitates better presentation of cell morphology and more complete cytologic evaluation.
Microbiologic evaluation of samples collected aseptically can be done if cytologic and clinical findings suggest an infectious agent is present. If possible, synovial fluid should be placed into a culture system immediately after collection. Use of an EDTA tube is undesirable because EDTA interferes with growth of some bacteria; a red-top tube is undesirable because it may not be sterile.
Laboratory Analysis and Reference Values
Volume
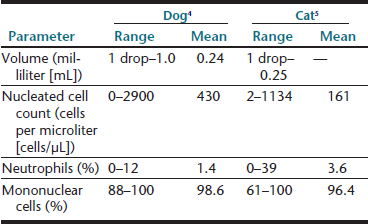
An approximate or subjective estimation of fluid volume collected should be recorded. Synovial fluid volumes depend on patient size and the joint from which it is being collected (within an individual, variation exists from joint to joint). In normal animals, fluid volume can range from 1 drop to 1 mL in dogs and 1 drop to 0.25 mL in cats.3–5 Clinical experience is an extremely valuable guide to detecting an articular effusion. This judgment is based on the degree of joint capsule distension, ease of fluid collection, and volume readily obtained. The aim of arthrocentesis for synovial fluid analysis is to collect some synovial fluid and not to drain the joint space.
Viscosity
Viscosity is easily assessed at the time of collection. However, if it must be evaluated after the sample is added to an anticoagulant, heparin is probably preferable to EDTA for sample preservation. EDTA tends to degrade hyaluronic acid and may decrease the sample’s viscosity.6
Viscosity may also be subjectively assessed when cytologically evaluating direct or sediment smears such that smears of fluids with normally high viscosity tend to have cells aligned in a linear pattern that is sometimes referred to as windrowing (Figure 12-9). In contrast, synovial fluid samples with decreased viscosity have cells more randomly arranged on the smear (Figure 12-10).

Figure 12-9 Direct smear of synovial fluid from a dog with acute suppurative arthritis.
Note the markedly increased cell count and linear arrangement of cells. The latter, referred to as windrowing, suggests normal viscosity.
(Wright stain. Original magnification 160×.) (From Parry BW: In Pratt PW, editor: Laboratory procedures for veterinary technicians, ed 2, Goleta, CA, 1992, American Veterinary Publications.)
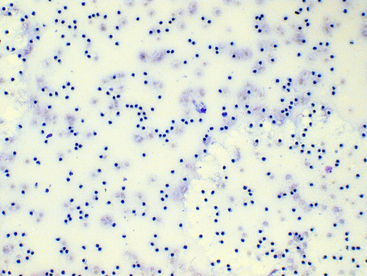
Figure 12-10 Direct smear of synovial fluid from a dog with suppurative (neutrophilic) polyarthritis.
The total nucleated cell count is increased and neutrophils are randomly distributed in the background, since the normal synovial fluid viscosity is degraded secondary to inflammation. (Wright-Giemsa stain. Original magnification 100×.)
Mucin Quality
If sufficient sample remains following slide preparation and nucleated cell count, synovial fluid mucin quality or hyaluronic acid may be assessed using a mucin clot test. When the potential exists for clotting of joint fluid, heparin is recommended as an anticoagulant because EDTA interferes with the mucin clot test by degrading hyaluronic acid.6
One part synovial fluid is added to four parts 2.5% glacial acetic acid, which causes mucin to precipitate and sometimes agglutinate or clot. The test is performed in test tubes when sufficient fluid is collected or on glass slides when only a drop is available for this test. The mixture is gently agitated and the nature of the clot observed. Assessment is enhanced by reading the test against a dark background. In inflammatory arthropathies, hyaluronic acid is degraded by proteases from neutrophils. This results in a decreased hyaluronic acid or hyaluronate concentration and decreased viscosity.
Total Cell Counts
Nucleated cell counts in normal synovial fluid vary from joint to joint within an individual animal.3 However, surveys have not shown these differences to be either statistically significant or clinically relevant. Various canine reference intervals have been reported (Table 12-3). As a generalization from these studies, most normal joints have nucleated cell counts less than 3000 cells/µL.
TABLE 12-3
REFERENCE INTERVALS FOR SYNOVIAL FLUID TOTAL NUCLEATED CELL COUNTS IN HEALTHY DOGS
| Range (cells/microliter [µL]) | Joints Sampled |
| 33–24953 | 12 joints: stifle, shoulder, carpus |
| 0–29004 | 55 joints: hip, stifle, hock, elbow, shoulder, carpus |
| 700–440012 | 20 stifles |
| 327–145013 | 14 stifles |
| 50–272514 | 58 stifles |
| 209–20707 | 19 stifles |
A recent study of synovial fluid samples from clinically normal cats, showed white blood cell (WBC) counts of 161 ± 209 cells/µL (mean ± standard deviation [SD]) and median WBC of 91 cells/µL with a range of 2 to 1134 cells/µL.5 Samples were excluded from this study when gross evidence of blood contamination was observed, radiographic evidence of osteoarthritis, or histologic evidence of synovitis was present or if postmortem physical examination revealed abnormalities. As a generalization from this study, most normal joints have nucleated cell counts less than 1000 cells/µL (Table 12-4).
A comparison of manual hemacytometer and electronic, automatic, particle counting of nucleated cells in canine synovial fluid revealed that the mean electronic total nucleated cell count was statistically higher than the mean manual count.7 Manual counting methods may demonstrate within-day and between-day analytic imprecision that is statistically higher than that of automated particle counting instruments.8,9 In general, differences in mean cell counts and precision have not proven to be clinically relevant; therefore, the efficiency and speed of automatic particle counters offer an advantage over manual methods.
EDTA is preferred as an anticoagulant and preservative for cytologic examination and nucleated cell counts. In comparison of EDTA and heparin anticoagulants as preservatives for synovial fluid, samples stored in heparin showed a fourfold and ninefold greater decrease in total nucleated cell counts over 24 hours and 48 hours at 4°C, respectively.8 In contrast, EDTA reportedly decreases synovial fluid mucin quality; therefore, total nucleated cell counts on fluid samples collected into EDTA may not be increased by hyaluronidase. Regardless of the anticoagulant or preservative used, synovial fluid should be pretreated with hyaluronidase when automated hematology analyzers are used for cell counts.10,11
Total Protein Concentration
Comparatively few studies have reported baseline values for total protein concentration, which probably reflects the relatively low priority given to this value. Other tests are preferred because sample volume is usually insufficient to allow for protein measurement. Synovial fluid protein concentration is best measured by a quantitative biochemical assay because refractometry measures other solutes and protein. A study of normal stifle, shoulder, and carpal joints reported a total protein concentration reference interval of 1.8 to 4.8 grams per deciliter (g/dL), as measured by the refractometer.3 Normal synovial fluid does not clot in vitro because it is essentially free of fibrinogen and other clotting factors. Joint fluid may form a thixolabile gel if left undisturbed for several hours. Because clots are not thixotropic, normal fluid is distinguishable from clotting by gently shaking the sample to restore fluidity. If a specimen forms a clot after collection, this indicates intraarticular hemorrhage or inflammation with increased vascular permeability and protein exudation into the joint space (Figure 12-12).
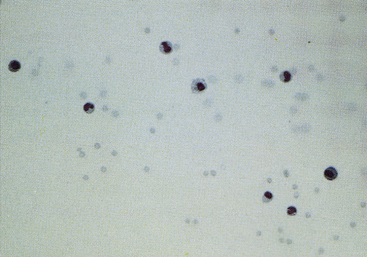
Figure 12-11 Sediment smear of synovial fluid from a dog with degenerative arthropathy.
Note the normal (low) cell count and random distribution of cells. The latter suggests decreased viscosity. (May-Grünwald-Giemsa stain. Original magnification 200×.)
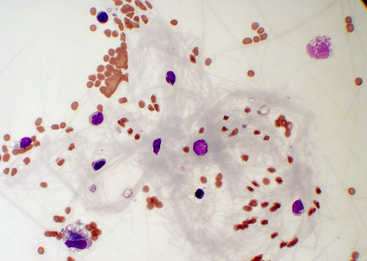
Figure 12-12 Synovial fluid from a cat with severe blunt-force trauma.
A fibrin clot in the fluid suggests hemorrhage or protein exudation into the joint space. Such a fibrin bundle can entangle nucleated cells and cause unpredictably altered total nucleated cell counts and aberrant white blood cell differentials. (Wright stain. Original magnification 500×.)
Cytologic Examination
Normal synovial fluid contains very few RBCs. Increased RBC numbers may result from hemorrhage associated with collection and trauma or inflammation involving the joint capsule. Generalizations regarding the cellularity of synovial fluid may be consistently made via freshly prepared direct smears. The body of the smear of normal specimens contains about 2 cells per field at 400× magnification (40× objective). Cellularity of direct smears are categorized as normal (Figure 12-11), slightly increased, moderately increased (Figure 12-13), or markedly increased (see Figure 12-9). Because of unpredictable variation among processing techniques and instrumentation, such assessments are impractical with concentrated specimens (sediment and cytocentrifuge smears).
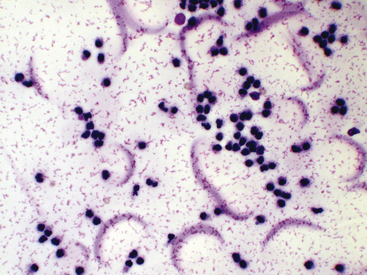
Figure 12-13 Synovial fluid from a cat with arthritis.
Fluid protein is observed as pink granular or stippled material and crescents. (Wright stain. Original magnification 500×.)
Because of the high viscosity of normal synovial fluid, cells of direct and centrifuged sediment smears tend to line up in rows (i.e., windrowing, see Figure 12-9). This characteristic arrangement may be used to comment on sample viscosity when volume is not sufficient for viscosity and mucin clot tests. However, in smears from synovial fluid with low cell counts, windrowing may not be apparent, even though viscosity is normal.
Smears of normal, and sometimes abnormal, synovial fluid may have a pink granular proteinaceous background (see Figures 12-13 and Figure 12-28), which must not be confused with bacteria.
Nucleated cells should be classified as neutrophils, large mononuclear cells, lymphocytes, or eosinophils. On poorly made slides, it may be difficult to differentiate collapsed neutrophils from lymphocytes (see Figure 12-13). Classification as mononuclear cells encompasses those that are phagocytically active. These cells could be derived from blood monocytes, tissues macrophages, or synovial lining cells. The origin of these cells has little practical importance with regard to clinical diagnosis and therapy. The proportion of large mononuclear cells that have phagocytized debris, cells, or microorganisms should be recorded. On smears that are freshly made or from fluid not exposed to EDTA, the degree of vacuolated large mononuclear cells should be noted and reported as mild, moderate, or marked. Sometimes, intact portions of intimal cells are directly sampled from the synovial lining, and a fraction of the large mononuclear component may have a spindle cell–like appearance. Observation of these spindloid cells may be seen in normal and diseased joints (Figure 12-14). Overall assessment of nucleated cell morphology ought to include comments on the degree of karyolysis, pyknosis, and karyorrhexis. Delayed processing may lead to nuclear degeneration and increased numbers of markedly vacuolated large mononuclear cells.3 Synovial fluid nucleated cell differentials are reported as percentage values and are incorporated into the interpretation of a total nucleated cell count or subjective assessment of cellularity.
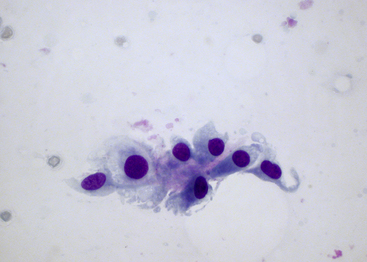
Figure 12-14 Synovial fluid from a dog without cytologic evidence of degenerative or inflammatory joint diseases.
An incidental observation included a cluster of intimal cells sampled from the synovial lining with a few of the large mononuclear cells demonstrating a spindle cell–like appearance. The scanty, amorphous, pink material embedded among the cells is likely intimal matrix or stroma. (Wright-Giemsa stain. Original magnification 500×.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree