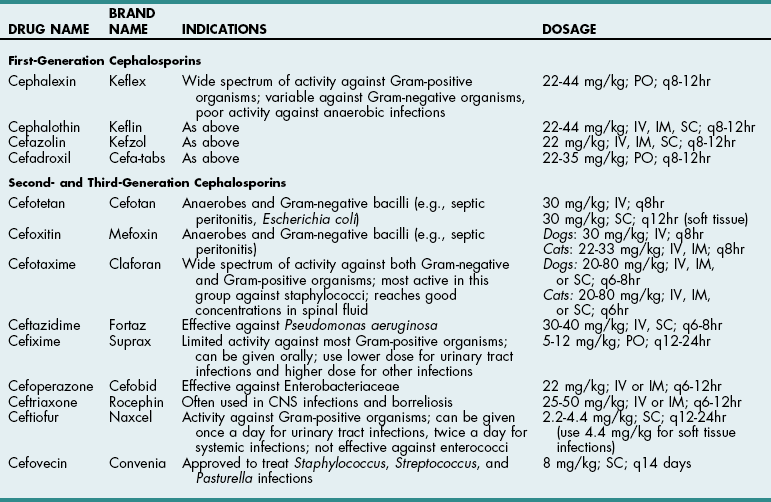
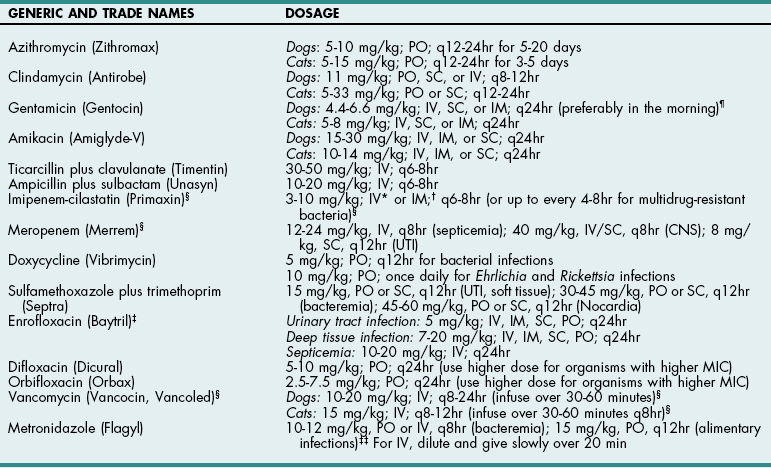
Chapter 9 Cephalosporins (Table 9-1) are generally more effective than penicillins against Gram-negative rods (e.g., Enterobacteriaceae), but they may be inactivated by cephalosporinases (a type of β-lactamase). Most are poorly effective against anaerobes (cefoxitin is an exception). First-generation cephalosporins are effective against most Gram-positive and some Gram-negative organisms. Second-generation cephalosporins have greater activity against Gram-negative bacteria and anaerobes but have no additional efficacy against Gram-positive organisms. Third-generation cephalosporins are highly effective against more than 90% of Gram-negative bacteria, but they often are less active against Gram-positive organisms than first-generation cephalosporins. Some third-generation cephalosporins have specific Gram-negative spectra, and it is important to note that just because one third-generation cephalosporin is effective for a particular infection does not mean that another third-generation cephalosporin will be effective. Ceftiofur is a third-generation cephalosporin with prolonged antibacterial activity because its major metabolite is active; however, it does not have a broad spectrum of activity against serious Gram-negative infections. Cefepime (Maxipime) is a fourth-generation cephalosporin that is unique among the cephalosporins because of its broad spectrum of activity, which includes Gram-positive cocci, enteric Gram-negative bacilli, and Pseudomonas aeruginosa. Cefovecin is an injectable, repositol cephalosporin developed to treat Gram-positive bacteria; it maintains therapeutic blood concentrations (depending on the bacteria being treated) for 7 to 14 days after being injected subcutaneously. Resistance to cephalosporins is mediated by the same mechanisms that cause resistance to penicillins. Cephalosporin Drugs Commonly Used in Veterinary Medicine PO, Oral; IV, intravenous; IM, intramuscular; SC, subcutaneous. Imipenem (Table 9-2) and aztreonam are β-lactam antibiotics that are highly resistant to β-lactamases. They are as effective against Gram-negative organisms as aminoglycosides but are not nephrotoxic. Imipenem (a carbapenem) has the broadest antibacterial spectrum of any systemic antimicrobial and is effective against most clinically relevant bacterial species, including Gram-negative and Gram-positive anaerobes and aerobes. It is not active against methicillin-resistant staphylococci or resistant strains of Enterococcus faecium. Because of their importance in human medicine as “drugs of last resort,” use of carbapenems should be restricted to severely ill patients who fail to respond to other antibiotics (see “Drugs of Last Resort” on p. 88). Aztreonam, a synthetic monobactam, is unaffected by bacterial β-lactamase. It is highly effective against many Gram-negative aerobes but has little activity against anaerobes. It has no activity against Gram-positive bacteria and must be used in combination with other drugs to achieve broad-spectrum activity. Dosages of New or Commonly Used Antibiotics in Veterinary Medicine *For infusion (imipenem-cilastatin injection), administer over 20 to 30 minutes. †For IM injection (suspension), reconstitute with 1% lidocaine. ‡When given IV, enrofloxacin is typically diluted and given over 10 to 20 minutes. Recent reports suggest that enrofloxacin may be associated with blindness in cats when doses greater than 5 mg/kg are used. It can be given as a single IV injection daily. §Considered “drugs of last resort.” Should only be used when extensive sensitivity testing shows that this drug is the only antibiotic to which the bacteria are sensitive. See discussion on “Drugs of Last Resort” in the chapter. ¶Doses up to 9-14 mg/kg may be given once daily in septicemia patients, for less than 7 days, renal function must be closely monitored. ‡‡As the total daily dose approaches 50 mg/kg, the risk for central vestibular problems increases. When using higher doses, it is best to treat for fewer than 2 weeks to avoid this. Erythromycin is readily absorbed from the upper gastrointestinal system and diffuses well throughout most tissues; however, it has a narrow spectrum of activity and may be associated with nausea and vomiting because of its prokinetic activity. New derivatives include clarithromycin (Biaxin), azithromycin (Zithromax), and dirithromycin (Dynabac). Azithromycin (see Table 9-2) is active against aerobic bacteria (e.g., staphylococci, streptococci, Helicobacter spp.) and anaerobes. It also has good activity against Mycoplasma spp., intracellular organisms (e.g., Bartonella spp., Toxoplasma spp.), and atypical mycobacteria. Oral absorption of azithromycin is high, and it is well tolerated. The drug achieves extremely high tissue concentrations and needs to be given only once daily. Fluoroquinolones (e.g., enrofloxacin, difloxacin, ciprofloxacin, ofloxacin, marbofloxacin) (see Table 9-2) and potentiated sulfas (e.g., trimethoprim-sulfa) inhibit DNA synthesis. Fluoroquinolones inactivate DNA gyrase, preventing uncoiling of the DNA molecule during DNA replication and transcription to messenger ribonucleic acid (mRNA). They are rapidly bactericidal and historically have been effective for soft tissue infections, pneumonia, osteomyelitis, and urinary tract infections caused by Gram-negative organisms and staphylococci. They also are effective against Rickettsia rickettsii and possibly L-form bacteria, but they are variably effective against Gram-positive cocci, especially enterococci (except staphylococci) and anaerobic bacteria. An additional reported advantage is activity against Pseudomonas aeruginosa, but reports suggest that higher than normal doses are required to achieve this effect. The dose of enrofloxacin varies depending on the target tissue (see Table 9-2). Possible side effects of quinolones include vomiting, CNS effects in animals of all ages, and cartilage and tendon lesions in developing animals. Like the aminoglycosides, the quinolones are concentration dependent, meaning that once-daily administration is typically preferred. In recent years, many isolates of pseudomonads, Escherichia coli, Enterococcus, and Staphylococcus spp. have become resistant to quinolones. In one human hospital, 80% of methicillin-resistant Staphylococcus aureus (MRSA) developed resistance to ciprofloxacin within 1 year of its introduction. In the United States and Europe, the prevalence of MRSA was less than 3% in the early 1980s but rose as high as 40% in the 1990s. Infections caused by MRSA have become a significant global health issue with serious consequences for all areas of human hospitals, especially operative rooms and intensive care units. Veterinary workers appear to be at an increased risk for colonization by MRSA, which might increase the rate of nosocomial infection in hospitalized patients although the risk to both staff and patients for clinical disease appears low (McLean and Ness, 2008; Walther et al, 2009). Methicillin-resistant Staphylococcus pseudintermedius (MRSP) has similar antibiotic resistance patterns and currently is of greater concern to most veterinarians than MRSA. MRSP rarely causes disease in people, and it infrequently causes extra-dermatologic infections in dogs or cats. The risk of MRSP transmitting the mecA gene (which causes methicillin resistance) to other bacteria is unclear, and this possibility has caused consternation among veterinarians. Indiscriminate use of antibiotics will continue to encourage the development of resistant strains in both human and veterinary hospitals.
Surgical Infections and Antibiotic Selection
Mechanisms of Antibiotic Action
Destruction of Bacterial Cell Walls
![]() TABLE 9-1
TABLE 9-1
![]() TABLE 9-2
TABLE 9-2
Inhibition of Protein Synthesis
Inhibition of DNA Synthesis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine
