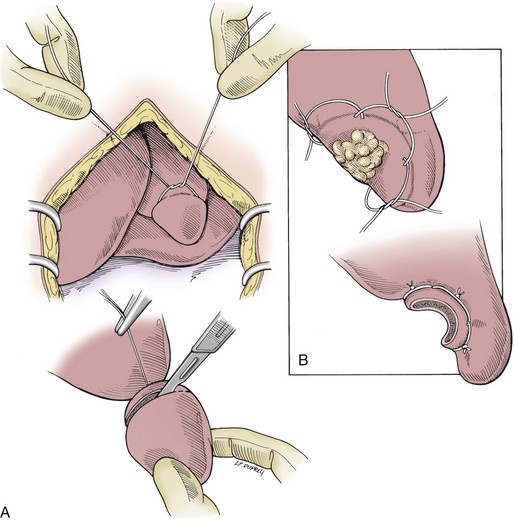
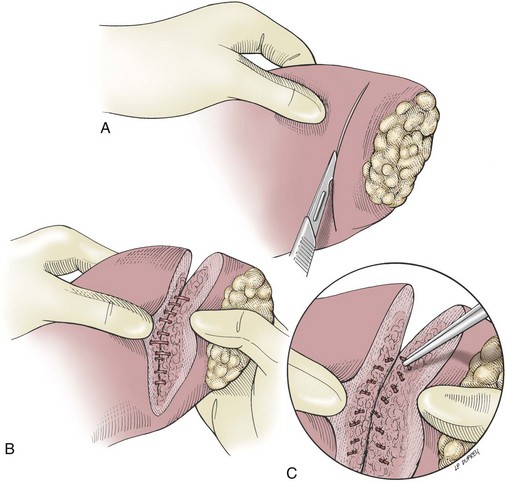
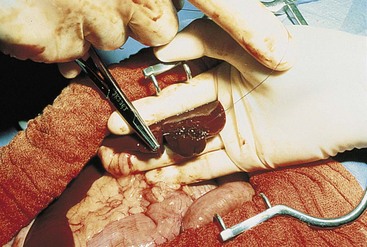
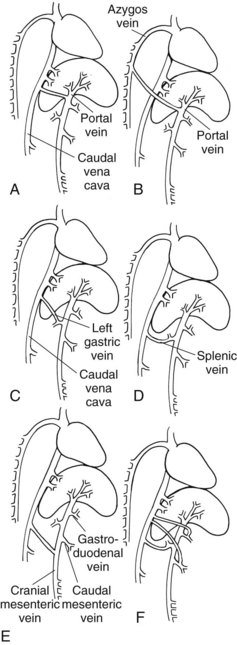
Chapter 21 General Principles and Techniques The liver is the largest gland in the body. It is the primary site for detoxification of many substances and plays a central role in the metabolism of protein, fat, and carbohydrates. Unfortunately, clinical signs of hepatic disease may not be apparent until the disease is advanced and irreversible. Hepatic failure may affect many other organ systems including the central nervous system (CNS), kidneys, intestines, and heart. A clinical scoring system based on the presence or absence of ascites, hypoglobulinemia, hepatic encephalopathy, hypoalbuminemia, hyperbilirubinemia, and clinical signs was associated with survival in Labrador Retrievers undergoing hepatic biopsy for chronic hepatitis (Shih et al, 2007). Higher clinical scores were negatively associated with survival in that study. Ascites in Labrador Retrievers presenting for chronic hepatitis was also associated with a shorter survival time (Raffan et al, 2009). The liver produces most of the plasma proteins (i.e., albumin, α- and β-globulins, fibrinogen, and prothrombin). Hypoalbuminemia is common in patients with advanced hepatic disease. Fluid therapy may further dilute serum albumin, making plasma or colloid infusions needed in severely affected patients. Albumin levels below 2 g/dl may be associated with delayed wound healing. Potassium abnormalities are common in patients with hepatic disease. Coagulopathies may occur because of diminished synthesis of clotting factors or consumption. Preoperative evaluation of clotting function, especially mucosal bleeding time, is warranted; fresh whole blood transfusions may reduce intraoperative hemorrhage in selected patients. Some patients with hepatic disease are anemic because of nutritional deficiencies, coagulation abnormalities, and/or gastrointestinal hemorrhage. Animals with hematocrits below 20% and anemic animals that are clinically hypoxic or weak should be given preoperative blood transfusions (see Table 4-5 and Box 4-1 on pp. 34 and 30, respectively). Many patients with hepatic disease are anorexic, and some require preoperative nutritional supplementation (see Chapter 10). Hypoglycemia sometimes occurs with severe hepatic insufficiency: blood glucose levels may need to be monitored. Patients with massive ascites may be dyspneic owing to pressure on the diaphragm that restricts lung expansion; removing some abdominal fluid before induction of anesthesia is important to prevent further hypoventilation and hypoxemia. If ascites prevents the patient from lying in dorsal recumbency, ventilation during surgery will be extremely difficult. Because high ventilator pressures reduce hepatic blood flow, it is important to reduce this pressure by removing some fluid; if time allows, it may be best to do this gradually over a couple of days. Patients with severe hepatic encephalopathy should be treated symptomatically (e.g., controlled protein diet, antibiotics, cleansing enemas, fluids, lactulose; see p. 601) to diminish or eliminate clinical signs before surgery. The goal of managing patients with hepatic dysfunction is to preserve what liver function is present while avoiding drugs or other factors that might be detrimental to it. Many medications normally administered during anesthesia undergo hepatic metabolism. In patients with moderate to severe dysfunction, such drugs should be avoided or used in markedly reduced doses. Furthermore, many medications are highly protein bound. With hypoalbuminemia, the percentage of bound drug is decreased and more is free in the plasma, resulting in more profound effects with normal drug dosages. Additionally, in patients with severe disease, alterations in the blood-brain barrier heighten the depressant effects of anesthetic medications (Table 21-1). Drugs to be avoided in animals with hepatic dysfunction include (but are not limited to) acepromazine, α2-agonists, pancuronium, vecuronium, telazol, and aminoglycosides. Premedications should not be administered to patients that are depressed. Midazolam is the preferred benzodiazepine in alert patients in which some sedation is warranted. Diazepam has active metabolites with a potentially very prolonged duration of action. It should be used only in patients with mild to moderate liver dysfunction and with no CNS depression. Because etomidate and propofol rely on redistribution rather than liver metabolism, they are the induction agents of choice. Etomidate, methohexital, and ketamine lower the seizure threshold. If recurrence of seizures is a risk, propofol should be administered for induction because of its antiepileptic effect. The sympathetic stimulation of ketamine makes it a less desirable induction agent, but its use at low doses may assist in lowering opioid requirements. Barbiturates should be used only in markedly reduced dosages. Likewise, the opioids are CNS depressants and must be used at reduced dosages. The opioid of choice during surgery and in the postoperative period is fentanyl because it can be titrated easily to effect as a continuous infusion. Early studies of morphine at doses 5 to 10 times normal recommended analgesic doses showed decreased hepatic portal blood flow. However, at normal doses and at the reduced doses required in hepatic dysfunction, this is not a concern. Morphine does cause histamine release with subsequent hypotension, usually occurring within 5 minutes of intravenous administration. If morphine is administered, hypotension should be anticipated and treated with a vasopressor. Usually, one to two doses is adequate because the hypotension is of short duration. Anesthetic Considerations for Animals With Hepatic Disease* *Patients with hepatic disease may have hypoalbuminemia with decreased binding as well as decreased clearance of many drugs. It is recommended to start with low doses and titrate slowly to effect. †Monitor for hyperthermia in cats. Because preservation of hepatic oxygenation has been shown to be extremely important in patients with liver dysfunction, hepatic blood flow must be maintained. Factors known to reduce hepatic blood flow, such as hypotension, excessive sympathetic stimulation (e.g., inadequate pain control), and high airway pressures, should be avoided by removal of ascites preoperatively. To increase oxygenation, preoxygenate before induction, keep inspired oxygen high during surgery, and do not rush extubation. Patients often need supplemental oxygen in the postoperative period. Hypotension needs to be treated promptly with vasopressors and/or inotropes (see Box 19-6 on p. 379). In patients with chronic liver disease and decreased liver mass, the circulatory system may be in a hyperdynamic state. Cardiac output is increased and is dependent on preload and systemic/pulmonary vasodilation. If chronic ascites has been present, some underlying renal dysfunction and impaired free water clearance may occur. Coupled with hypoalbuminemia, colloids are often warranted instead of large boluses of crystalloids. Fluid maintenance needs to be individualized based on underlying causes, severity of hepatic and renal dysfunction, degree of anemia and hypoalbuminemia, blood loss in surgery, and evaporative losses due to incision size. As a general rule, replace evaporative losses with crystalloids and blood loss with colloids to assist in maintaining adequate blood pressure. See recommendations on p. 602 for anesthetic management of animals with portosystemic shunts. Aerobic and anaerobic bacteria normally reside in the liver but may proliferate with hepatic ischemia or hypoxia. Therefore, prophylactic antibiotics are warranted in patients with severe hepatic disease that are undergoing hepatic surgery besides simple biopsy. The pharmacokinetics of antibiotics may be altered in these patients by depressed hepatic metabolism, alterations in hepatic arterial or portal blood flow, hypoalbuminemia, or reductions in biliary excretion. Antibiotics are specifically indicated in the treatment of hepatic encephalopathy (see p. 601), bacterial cholangitis/cholangiohepatitis, and hepatic abscess. Broad-spectrum antibiotics effective against anaerobes (e.g., penicillin derivatives, metronidazole, clindamycin) are appropriate and relatively safe in patients with hepatocellular compromise (Box 21-1). Potentially hepatotoxic antibiotics (e.g., chlortetracycline, erythromycin) should be avoided if possible. The diaphragmatic surface (parietal surface) of the liver is convex and lies mainly in touch with the diaphragm. The visceral surface faces caudoventrally and to the left and contacts the stomach, duodenum, pancreas, and right kidney. Six hepatic lobes are present (Fig. 21-1). The borders of the liver are normally sharp but appear more rounded in young animals and in those with infiltrated, congested, or scarred livers. The liver has two afferent blood supplies: a low-pressure portal system and a high-pressure arterial system. The portal vein drains the stomach, intestines, pancreas, and spleen and supplies four-fifths of the blood that enters the liver. The remainder of the afferent blood supply is derived from the proper hepatic arteries. These arteries are branches of the common hepatic artery and may number between two and five. Efferent drainage of the liver occurs through the hepatic veins. In the fetal pup, the ductus venosus shunts blood from the umbilical vein to the hepatic venous system. The ductus venosus becomes fibrotic after birth and is known as the ligamentum venosum. Bile, formed in the liver, is discharged into bile canaliculi lying between the hepatocytes. These canaliculi unite to form interlobular ducts that ultimately merge to form lobar or bile ducts (see p. 619). The portal vein, bile ducts, hepatic artery, lymphatics, and nerves are contained in the lacelike and nonsupporting portion of the lesser omentum known as the hepatoduodenal ligament. Hepatic biopsies are commonly indicated in patients known to have or suspected of having hepatic disease. Several breeds have been associated with hepatopathies, including Bedlington Terrier, Doberman Pinscher, Cocker Spaniel, West Highland White Terrier, Dalmatian, Skye Terrier, and, recently, Labrador Retriever (Shih et al, 2007; Poldervaart et al, 2009). Chronic hepatitis of Labrador Retrievers occurs in middle-aged to older dogs; it causes no or vague clinical signs and is likely associated with copper accumulation (Hoffmann et al, 2009). Anorexia, hypoglobulinemia, and prolonged partial thromboplastin time (PTT) were associated with shorter survival; however, the median survival was 1 year, with some affected dogs surviving as long as 8 years. Biopsies may be obtained percutaneously, with laparoscopy (see p. 590), or at surgery. Partial hepatectomies are less commonly performed but may be indicated for focal neoplasms or trauma. The standard approach for hepatic surgery is a cranial ventral midline abdominal incision. The caudal aspect of the sternum can be split if additional exposure is needed. Tissue core biopsies may be obtained with a Tru-Cut biopsy (Cardinal Health, Dublin, Ohio) (Fig. 21-2), a large-bore needle, or an automated biopsy device (e.g., Bard Biopsy instrument, Bard Biopsy Systems, Tempe, Ariz.). The latter should not be used in cats because of potential mortality associated with the shock wave caused by triggering the device. For histopathologic specimens, the Tru-Cut needle should be removed from the syringe or gun and placed in formalin. Once the sample has been fixed, it should be removed from the needle for processing. A core biopsy should use the largest-gauge needle that may be used safely in the patient, typically a 14 gauge needle. If core biopsies are performed, at least two or three (2 cm long) samples should be obtained. Core biopsies generally are taken from only one liver lobe (i.e., the left liver lobe, so as to minimize the chance of lacerating the bile ducts or gallbladder, both of which are on the right side). However, this is a significant limitation because lesions may be present in only a few of the liver lobes. Finally, extreme care must be taken to ensure that the core biopsy needle will not pass through the liver and lacerate structures (e.g., veins, stomach, intestines, diaphragm, lungs, heart) under the hepatic lobe being biopsied. Fine needle aspiration is most likely to be diagnostic in patients with diffuse hepatic neoplasia (e.g., lymphosarcoma, diffuse hepatic histoplasmosis, feline idiopathic hepatic lipidosis). However, inability to diagnose these conditions on a fine needle aspirate does not preclude disease, and even if one of these conditions is found, other, undiagnosed diseases could be present. This latter possibility is particularly important in cats, because almost all sick, anorexic cats will have some fat vacuoles in the hepatocytes. However, before clinical illness due to hepatic lipidosis can be diagnosed, one must determine that fat in the hepatocytes is sufficient to cause hepatic dysfunction. Furthermore, diagnosing hepatic lipidosis does not guarantee that there is not another hepatic disease present that was not found by the fine needle technique. Cytologic evaluation of ultrasound-guided fine needle aspirates is most likely to agree with histopathologic findings when the animal has vacuolar hepatopathy; however, this condition is commonly misdiagnosed by cytology. In one study, overall agreement between cytology and histopathology in dogs and cats was 30.3% and 51.2%, respectively (Wang et al, 2004). In another study, in which morphologic diagnoses were made with the use of an 18 gauge Tru-Cut–type needle, concurrence with wedge biopsy specimens was approximately 50% in both dogs and cats (Cole et al, 2002). The substantial limitations of diagnosing hepatic disease in dogs and cats by percutaneous techniques should be recognized by clinicians. Percutaneous biopsies may be obtained under tranquilization or heavy sedation. Approximately 55.5% of cats required injectable anesthesia for needle biopsy to be performed in one study (Proot and Rothuizen, 2006). Samples are obtained using a transthoracic or transabdominal approach; however, to avoid laceration of the liver during respiration, the former should be utilized only if the latter is not possible. The latter is described here. With the animal in dorsal recumbency, clip the hair from the area surrounding the xiphoid process and prepare it for aseptic surgery. Make a small incision in the skin on the left side between the costal arch and the xiphoid process. Insert the biopsy needle through the skin incision in a craniodorsal direction, angling it slightly toward the left of midline. Advance the needle until ultrasound guidance shows the needle to be positioned at the surface of the liver. Advance the biopsy needle into the hepatic tissue, and obtain the biopsy sample (see Fig. 21-2). Three ultrasound-guided methods can be used to biopsy or aspirate hepatic structures or lesions. Any of these techniques may be used when samples of the liver are obtained. If diffuse disease is suspected, the left liver should be sampled to avoid the main biliary structures. Extreme care should be taken to ensure that no large biliary or vascular structures lie within the 2 cm area required for needle advancement (Proot and Rothuizen, 2006). The first technique is called the indirect guidance method and is not generally recommended. It entails using ultrasound to find the structure of interest, then the ultrasound probe is removed. The needle is passed blindly in the area of interest. This technique is applicable only when the target is extremely large and direct visualization of the needle is not required as it is passed into the structure to be biopsied or aspirated. Complications associated with an automated needle biopsy device in one study in cats led to elimination of its use and integration of a semi-automated biopsy needle device for sample procurement (Proot and Rothuizen, 2006). Death from shock occurred in 19% of cats undergoing automated Tru-Cut biopsy, whereas none of the subsequent 19 cases in which the semi-automated Tru-Cut biopsy device was used had complications. The authors speculated that a strong increase in vagal tone due to the force of the automated device led to severe bradycardia, weak peripheral pulses, respiratory impairment, loss of consciousness, and cardiac arrest. Laparoscopy (see Chapter 13) offers several advantages over other hepatic biopsy techniques. First, laparoscopy can find lesions missed by ultrasound, allowing the clinician to biopsy clearly abnormal areas that would have been missed if a percutaneous technique had been used. Second, laparoscopy allows one to obtain better tissue samples than is possible with percutaneous techniques (sufficient hepatic tissue can readily be obtained for histopathology, mineral analysis, and culture), and it allows multiple liver lobes to be biopsied (as opposed to just one lobe, as commonly occurs with core needle techniques). Laparoscopic biopsy using a 5 mm double spoon biopsy forceps should obtain approximately 45 mg of tissue. In comparison, an 18 gauge Tru-Cut will obtain approximately 5 mg of tissue, and a 14 gauge Tru-Cut will obtain approximately 15 to 20 mg of tissue. At the same time, the endoscopist can quickly examine the abdomen and peritoneum, omentum, stomach, pancreas, intestines, and/or kidneys to see if any other, unsuspected disease is present. Finally, laparoscopy can be done quickly (i.e., in less than 20 minutes), and the patient routinely is able to be discharged within hours of completion of the procedure. Coagulopathies are not an absolute contraindication unless they are severe; electrocautery and coagulation-enhancing materials can be used but are seldom needed. Alternatively, use a harmonic scalpel to harvest liver samples. Collect a 2 × 1 cm sample of liver using a harmonic scalpel with a working blade length of 15 mm and set to a power level of 3 (Barnes et al, 2006). Hemorrhage routinely occurs when the sample is torn off the liver, but one must be aware that the laparoscope will magnify any hemorrhage that occurs. Typically, less than 1 to 5 ml of blood is lost with a biopsy. Less hemorrhage was noted when the liver was sampled with the harmonic scalpel; however, significantly more coagulation necrosis, cavitational fragmentation, and fibrosis were associated with the harmonic scalpel (Barnes et al, 2006; Vasanjee et al, 2006). Although less than 25% or up to 1.3 mm of the sample was necrotic, appropriately sized samples should be obtained to offset the tissue damage. Sampling from central regions of the liver with the harmonic scalpel seemed to rely on natural coagulation rather than vessel sealing (Vasanjee et al, 2006). Needle biopsies least often result in adequate samples for histopathologic evaluation. A centrally located sample of liver parenchyma may be taken with a Baker biopsy punch. The recommended size for collection of at least six to eight portal triads is 6 mm in diameter. Press the punch against the area to be sampled and advance it using a clockwise and counterclockwise twisting motion (Vasanjee et al, 2006). Use Metzenbaum scissors to separate the deep margin of the sample from the parenchymal attachment. If needed, insert hemostatic gelatin sponge material into the defect to aid in hemostasis. A biopsy of the hepatic margin may be obtained by the “guillotine” method. Place a loop of suture around the protruding margin of a liver lobe. Pull the ligature tight and allow it to crush through the hepatic parenchyma before tying it (Fig. 21-3, A). As the suture tears through the soft hepatic tissue, vessels and biliary ducts are ligated. Hold the liver gently between the fingers, and with a sharp blade, cut the hepatic tissue approximately 5 mm distal to the ligature (allowing the stump of crushed tissue to remain with the ligature). To avoid crushing the biopsy sample and causing artifacts, do not handle it with tissue forceps. Place a portion of the sample in formalin for histologic examination; reserve the remainder for culture and cytologic study. Check the biopsy site for hemorrhage. If hemorrhage continues, place a pledget of absorbable gelatin foam over the site. As an alternative, if a focal (nonmarginal) area of the liver is to be biopsied, use a punch biopsy or a Tru-Cut biopsy (see Fig. 21-2), or place several overlapping guillotine sutures around the margin of the lesion and excise it (Fig. 21-3, B). Use caution with a punch biopsy to avoid penetrating more than half the thickness of the liver with each biopsy. Apply pressure to the site until bleeding stops. If hemorrhage continues, place a pledget of absorbable gelatin sponge over the site. Determine the line of separation between normal hepatic parenchyma and that to be removed, and sharply incise the liver capsule along the selected site (Fig. 21-4, A). Bluntly fracture the liver with the fingers (Fig. 21-4, B) or the blunt end of a Bard-Parker scalpel handle, and expose the parenchymal vessels. Ligate large vessels (hemoclips may be used), and electrocoagulate small bleeders encountered during the dissection (Fig. 21-4, C). As an alternative, place a stapling device (Autosuture TA 90, 55, or 30; Ethicon, Somerville, N.J.) across the base of the lobe and deploy the staples. Excise the hepatic parenchyma distal to the ligatures or staples. Before closing the abdomen, make sure the raw surface of the liver is dry and free of hemorrhage. In small dogs and cats, several overlapping guillotine sutures (as described previously) may be placed along the entire line of demarcation (Fig. 21-5). Be sure that the entire width of the hepatic parenchyma is included in the sutures. After tightening the sutures securely, use a sharp blade to cut the hepatic tissue distal to the ligature, allowing a stump of crushed tissue to remain with the ligature. Partial lobectomy in medium-sized dogs (24.3 kg) has been described using a single pre-tied loop ligature of 0 glycolide-lactide copolymer, a vessel-sealing device (see p. 83), and a harmonic scalpel (Risselada et al, 2010). Intraoperative hemorrhage occurred with pre-tied loops and the harmonic scalpel, resulting in additional ligation or clip application. None of the three methods leaked upon postmortem perfusion until supraphysiologic pressures in the hepatic artery or portal vein were reached, suggesting that further investigation into these techniques may be warranted (Risselada et al, 2010). Dissection of individual liver lobes can be achieved for selective excision (Covey et al, 2009). Each may be drained by more than one hepatic vein, and careful identification of the venous drainage can be achieved using the central portion of a Poole suction tip. The hepatic vein(s) associated with the left lateral or left medial liver lobe may be closed using a thoracoabdominal stapling device after ligation and transection of the hepatic artery and biliary duct associated with the lobe to be removed. Recovery from anesthesia should be monitored closely in animals with severe hepatic dysfunction. Because of the increased half-life of some drugs in patients with hepatic dysfunction, recovery may be prolonged. Intravenous fluids should be provided until the patient is able to maintain hydration, but care must be taken to avoid overhydrating hypoalbuminemic patients. Blood glucose levels should be monitored; transient hypoglycemia is common after removal of large portions of the liver. Hypophosphatemia may occur after lobectomy and may require supplementation of potassium phosphate in the IV fluids. Albumin levels should be monitored. If the patient becomes severely hypoalbuminemic (i.e., less than 2 g/dl) or has substantial worsening of third space fluid accumulation, one should consider administering plasma, whole blood, or synthetic colloids (e.g., hetastarch). Clotting times may be assessed if hemorrhage or petechiation occurs. Antibiotics given during surgery should be continued for 2 to 3 days if partial hepatectomy has been performed. Nutritional supplementation may be necessary in some patients during the early postoperative period, particularly if the animal is anorexic or has severe vomiting or diarrhea (see Chapter 10). Analgesics (e.g., hydromorphone, butorphanol, buprenorphine) should be provided to patients after surgery (see Table 12-3 on p. 141). For severe pain, a fentanyl-lidocaine-ketamine combination given as a constant rate infusion (CRI) may be indicated (see Box 12-2 on p. 138). Dogs with acute hepatitis may progress to chronic hepatitis and cirrhosis; repeat liver sampling should be considered to assess response to therapy (Poldervaart et al, 2009). The most common and serious complication of hepatic surgery is hemorrhage. This may result from ligatures slipping off friable hepatic tissue. Care should be taken to ensure that a stump of tissue remains distal to the ligature when encircling sutures are used for biopsy or partial hepatectomy. With hepatic trauma, anaerobic bacteria may proliferate in hypoxic portions of the liver and cause sepsis; therefore, broad-spectrum antibiotics should be used in patients with severe hepatic trauma and in those undergoing hepatic surgery. Complications after major hepatic resection include portal hypertension, ascites, fever, hemorrhage, hypophosphatemia, or persistent bile drainage. Dogs with ascites undergoing hepatic biopsy have a significantly shorter mean survival time than those without ascites (0.4 m and 24 m from the time of biopsy, respectively) (Raffan et al, 2009). Icterus, hypoalbuminemia, a left shift on the leukogram, and enlarged portal lymph nodes were also associated with shorter survival in dogs with primary hepatitis (Poldervaart et al, 2009). Portosystemic shunt ligation (see p. 601) is often performed in young animals that are particularly prone to hypoglycemia; serum glucose concentrations should be carefully monitored. Hypothermia, also a particular problem in young patients, reduces the MAC of inhalants used for anesthetic maintenance. Barnes, RF, Greenfield, CL, Schaeffer, DJ, et al. Comparison of biopsy samples obtained using standard endoscopic instruments and the harmonic scalpel during laparoscopic and laparoscopic-assisted surgery in normal dogs. Vet Surg. 2006;35:243. Cole, TL, Center, SA, Flood, SN, et al. Diagnostic comparison of needle and wedge biopsy specimens of the liver in dogs and cats. J Am Vet Med Assoc. 2002;220:1483. Covey, JL, Degner, DA, Jackson, AH, et al. Hilar liver resection in dogs. Vet Surg. 2009;38:104. Hoffmann, G, Jones, PG, Biourge, V, et al. Dietary management of hepatic copper accumulation in Labrador retrievers. J Vet Intern Med. 2009;23:957. Poldervaart, JH, Favier, RP, Penning, LC, et al. Primary hepatitis in dogs: a retrospective review (2002-2006). J Vet Intern Med. 2009;23:72. Proot, SJM, Rothuizen, J. High complication rate of an automatic Tru-Cut biopsy gun device for liver biopsy in cats. J Vet Intern Med. 2006;20:1327. Raffan, E, McCallum, A, Scase, TH, et al. Ascites is a negative prognostic indicator in chronic hepatitis in dogs. J Vet Intern Med. 2009;23:63. Risselada, M, Polyak, MM, Phil, M, et al. Postmortem evaluation of surgery site leakage by use of in situ isolated pulsatile perfusion after partial liver lobectomy in dogs. Am J Vet Res. 2010;71:262. Shih, JL, Keating, JH, Freeman, LM, et al. Chronic hepatitis in Labrador retrievers: clinical presentation and prognostic factors. J Vet Intern Med. 2007;21:33. Vasanjee, SC, Bubenik, LJ, Hosgood, G, et al. Evaluation of hemorrhage, sample size, and collateral damage for five hepatic biopsy methods in dogs. Vet Surg. 2006;35:86. Wang, KY, Panciera, DL, Al-Rukibat, RK, Radi, ZA. Accuracy of ultrasound-guided fine-needle aspiration of liver and cytologic findings in dogs and cats: 97 cases (1990-2000). J Am Vet Med Assoc. 2004;224:75. Specific Diseases PSSs are broadly categorized as congenital or acquired, and as intrahepatic or extrahepatic. Congenital extrahepatic shunts typically are single anomalous vessels that allow abnormal blood flow from the portal vein directly to the systemic circulation; rarely two or more anomalous vessels may be present. CEPSS accounts for nearly 63% of single shunts in dogs; it also occurs in cats. Many different types of CEPSS have been described in dogs, including (1) portal vein to caudal vena cava, (2) portal vein to azygos vein, (3) left gastric vein to caudal vena cava, (4) splenic vein to caudal vena cava, (5) left gastric, cranial mesenteric, caudal mesenteric, or gastroduodenal vein to caudal vena cava, and (6) combinations of the above (Fig. 21-6). Cats most commonly have a large single vessel that empties directly into the prehepatic vena cava; however, they may have atypical CEPSS connections, and the shunt may flow into any systemic vessel including renal, phrenicoabdominal, azygos, or internal thoracic veins. Intrahepatic shunts usually are congenital, singular shunts that occur because the ductus venosus fails to close after birth, or they may arise when other portal to hepatic vein or caudal vena cava anastomoses exist. Congenital IHPSSs make up about 35% of single shunts in dogs and approximately 10% in cats. Acquired extrahepatic shunts typically are multiple and represent about 20% of all canine PSSs. They arise in part because of increased resistance to portal blood flow causing portal hypertension with a pressure gradient between portal and systemic circulation. This hypertension causes normal, nonfunctional microvascular connections that are present at birth between portal and systemic veins to become functional. Multiple shunts are most commonly associated with chronic, severe hepatic disease (i.e., cirrhosis) but have been reported secondary to hepatoportal fibrosis in young dogs. Veno-occlusive hepatic disease has been reported as a cause of multiple PSSs in young Cocker Spaniels. Multiple shunts most commonly occur in the left renal area and the root of the mesentery (Fig. 21-7), and connections to the caudal vena cava or azygos veins usually are seen. Cats with acquired PSSs typically develop connections between the left gastric veins and the phrenicoabdominal veins, and from the left colic vein to the ipsilateral gonadal vein. FIG 21-7 Multiple shunts near the left kidney in a dog with hepatic disease and portal hypertension. Arteriovenous (A-V) fistulae account for about 2% of single shunts and may be congenital or acquired. Acquired A-V fistulae occur secondary to trauma, tumors, surgical procedures, or degenerative processes that cause arteries to rupture into adjacent veins. The fistulae typically are macroscopic communications that form between branches of the hepatic artery and portal vein; however, microscopic hepatic A-V fistulae have also been suspected. As congenital lesions, they are believed to develop as a result of failure of the common embryologic capillary plexus to differentiate into an artery or a vein. Affected animals usually develop portal hypertension and multiple collateral shunting vessels, resulting in acute onset of low-protein transudative ascites between the ages of 2 and 18 months (Figs. 21-8 and 21-9). In contrast, dogs with congenital PSS rarely have ascites.
Surgery of the Liver
Preoperative Concerns
Anesthetic Considerations
![]() TABLE 21-1
TABLE 21-1



Antibiotics
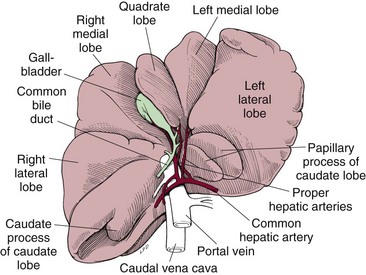
Surgical Anatomy
Surgical Techniques
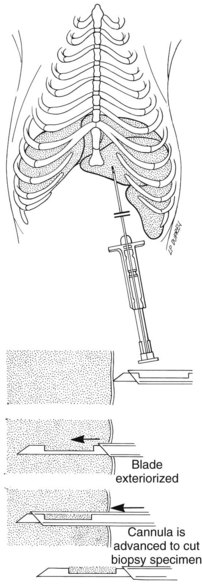
Percutaneous Liver Biopsy
Percutaneous blind biopsy
Percutaneous ultrasound-guided biopsy
Laparoscopic Liver Biopsy
Surgical Liver Biopsy
Partial Lobectomy
Complete Lobectomy
Postoperative Care and Assessment
Complications
Special Age Considerations
References
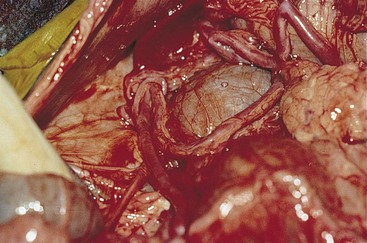
Portosystemic Vascular Anomalies
General Considerations and Clinically Relevant Pathophysiology
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgery of the Liver

 to
to  minimum alveolar concentration (MAC) is needed.
minimum alveolar concentration (MAC) is needed.