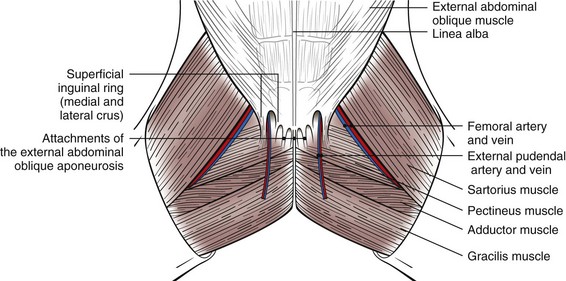
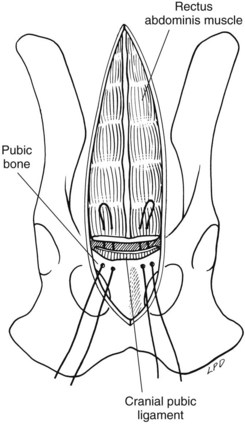
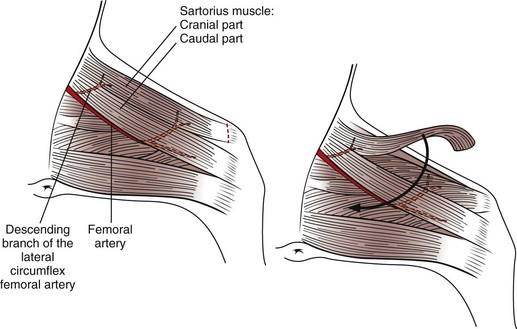
Chapter 19 General Principles and Techniques The decision to operate is based on the history and physical examination findings, radiographic and ultrasonographic studies, and laboratory analyses. Physical examination can be unreliable in predicting the severity of abdominal trauma. The inaccuracy associated with examining patients with acute abdominal disease, particularly that associated with trauma, can be attributed in part to the patient’s condition at the time of examination and delayed development of clinical signs associated with some injuries. Depressed or lethargic animals may not show pain during abdominal palpation. Clinical signs of hemorrhage often are inapparent immediately after trauma; delays of 3 to 4 hours between injury and development of shock and collapse are common in patients with hepatic or splenic lacerations. Therefore, animals that have suffered traumatic injury should be closely observed for at least 8 to 12 hours. Life-threatening hemorrhage becomes apparent before this time in most cases. However, animals with traumatic bilious peritonitis may not have overt clinical signs for weeks. Likewise, traumatic mesenteric avulsion is seldom associated with clinical signs until peritonitis develops, usually several days after injury. Sensitive diagnostic tests such as diagnostic peritoneal lavage (see p. 380) may help identify patients with significant abdominal trauma before overt clinical signs develop. Preoperative management of most animals undergoing exploratory laparotomy is dictated by the underlying abdominal disease. General observations include noting the animal’s attitude and posture, temperature, respiratory rate and effort, and heart rate and rhythm. Abdominal auscultation, percussion, and palpation plus rectal examination are indicated. Serial examinations are important to detect trends or deterioration in the patient’s status. An intravenous catheter should be placed for fluid and drug administration, and blood samples should be drawn. Useful initial blood work in an animal with acute abdomen includes complete blood count (CBC), platelet count, serum total protein and glucose concentrations, and blood urea nitrogen (BUN). Other laboratory tests (e.g., serum biochemistry profile, clotting parameters) can be performed, depending on the animal’s condition and the suspected underlying disease. Urine may be collected by means of cystocentesis or catheterization for urinalysis. An indwelling urinary catheter may be used to quantitate urinary output if necessary. Abdominal radiographs may detect peritoneal fluid (i.e., uroabdomen, peritonitis) or abnormal accumulations of air. Animals with acute abdominal signs of uncertain cause should have abdominocentesis, diagnostic peritoneal lavage (see p. 380) FAST (see p. 383), or a CT performed if radiographs are nondiagnostic. Electrolyte and hydration abnormalities should be corrected before surgery. Major abdominal evisceration may occur in dogs secondary to postsurgical dehiscence or trauma. In affected animals, the intestines typically are eviscerated and may have gross contamination with dirt or other debris (e.g., cat litter). Regardless of the inciting cause, exposure and contamination of the abdominal viscera warrant immediate surgical intervention. In a recent study of animals that had developed a postoperative major abdominal evisceration, all had recently undergone ovariohysterectomy (Gower et al, 2009). The authors noted that the preponderance of wound dehiscence leading to abdominal evisceration in animals undergoing ovariohysterectomy was likely a reflection that it is one of the most frequently performed abdominal procedures in small animals, rather than being a direct consequence of the procedure. The anesthetic management of animals with abdominal disease depends on the underlying disease. Animals that are not in shock can be premedicated with a benzodiazepine and opioid and induced with propofol, ketamine, or etomidate given intravenously to effect (Table 19-1). Table 19-2 provides suggested anesthetic protocols for animals that are in shock or that are debilitated. Anesthetic Considerations in the Stable Patient Undergoing Abdominal Surgery *Monitor for hyperthermia in cats. †Buprenorphine is a better analgesic than morphine in cats. ‡Only use in young, healthy animals. §Black box warning added by the FDA in October 2010 identified cases of renal failure and death in cats with repeated uses of meloxicam. Meloxicam is approved for single use only in cats in the U.S. Appropriate use of antibiotics in patients undergoing abdominal surgery depends on the underlying disease, the animal’s overall general health, and the length and type of surgical procedure performed (see Chapter 9). The rectus sheath is composed of an external and an internal leaf (Fig. 19-1). The external leaf is formed by the aponeurosis of the external abdominal oblique muscle and a portion of the aponeurosis of the internal abdominal oblique muscle. The aponeurosis of the transversus abdominis muscle joins the external leaf near the pubis (see Fig. 19-1). The internal leaf consists of a portion of the aponeurosis of the internal abdominal oblique muscle, the aponeurosis of the transversus abdominis muscle, and the transversalis fascia. The internal leaf disappears in the caudal third of the abdomen, where the aponeurosis of the internal abdominal oblique muscle joins the external leaf, leaving the caudal rectus abdominis muscle covered only by a thin sheet of transversalis fascia and peritoneum (see Fig. 19-1). With the patient in dorsal recumbency, make a ventral midline skin incision beginning near the xiphoid process and extending caudally to the pubis (Fig. 19-2, A). Sharply incise the subcutaneous tissues until the external fascia of the rectus abdominis muscle is exposed. Ligate or cauterize small subcutaneous bleeders and identify the linea alba. Tent the abdominal wall and make a sharp incision into the linea alba with a scalpel blade. Palpate the interior surface of the linea for adhesions. Use scissors to extend the incision cranially or caudally (or both) to near the extent of the skin incision. Digitally break down the attachments of one side of the falciform ligament to the body wall, or excise it and remove it entirely if it interferes with visualization of cranial abdominal structures. Clamp the cranial end of the falciform ligament and ligate or cauterize bleeders before removing it. With the patient in dorsal recumbency, place a towel clamp on the prepuce and clamp it to the skin on one side of the body (Fig. 19-2, B). Drape the tip of the prepuce and clamp outside the surgical field. Make a ventral midline skin incision beginning at the xiphoid process and continuing caudally to the prepuce. Curve the incision to the left or right of the penis and prepuce (i.e., the side opposite the clamped prepuce) and extend it to the level of the pubis (see Fig. 19-2, B). Incise the subcutaneous tissues and fibers of the preputialis muscle to the level of the rectus fascia in the same plane as the skin incision. Ligate or cauterize large branches of the caudal superficial epigastric vein at the cranial aspect of the prepuce. Retract incised skin and subcutaneous tissues laterally and locate the linea alba and external fascia of the rectus abdominis muscle. Do not attempt to locate the caudal linea alba until subcutaneous tissues have been incised and the abdominal musculature fascia identified. Tent the abdominal wall and make a sharp incision into the linea alba with a scalpel blade. Palpate the interior surface of the linea for adhesions. Use scissors to extend the incision cranially or caudally (or both) to near the extent of the skin incision. Systematically explore the entire abdomen. Various techniques may be used; however, every surgeon should develop a consistent pattern to ensure that the entire abdominal cavity and all structures in each animal are visualized and/or palpated (Box 19-1). On each side of the incision, incorporate 4 to 10 mm of fascia in each suture. Place interrupted sutures 5 to 10 mm apart, depending on the animal’s size. Tighten sutures sufficiently to appose but not enough to strangulate tissue, because too-tight sutures adversely affect wound healing. Incorporate full-thickness bites of the abdominal wall in the sutures if the incision is midline (i.e., through the linea alba; Fig. 19-3). Do not incorporate the falciform ligament between the fascial edges. If the incision is lateral to the linea alba and muscular tissue is exposed (i.e., paramedian incision), close the external rectus sheath without including muscle in the sutures. Do not attempt to include peritoneum in the sutures. Close subcutaneous tissues with a simple continuous pattern of absorbable suture material, and reappose the preputialis muscle fibers. Use nonabsorbable sutures (simple interrupted or continuous appositional pattern; see Chapter 8) or stainless steel staples to close the skin. Place skin sutures without tension. The abdominal incision should be checked twice daily for redness, swelling, or discharge. If the animal licks or chews at the incision, an Elizabethan collar or sidebar should be used to prevent iatrogenic suture removal. Early signs of altered wound healing include inflammation and edema. Swelling and serosanguineous drainage from the incision are consistent signs of acute incisional dehiscence. Dehiscence usually occurs 3 to 5 days after surgery, when minimal healing has occurred and the sutures have weakened; however, it may occur earlier if knots were tied improperly or if fascia was not incorporated into the sutures. Evisceration usually causes sepsis and severe blood loss secondary to mutilation of exposed intestine; the patient must be treated promptly. The abdomen should be bandaged, fluid therapy initiated, and broad-spectrum antibiotics given while the animal is prepared for surgery. If technical failure such as poor knot tying or improper suturing is suspected, the entire suture line should be removed and replaced. Débridement of the wound edges is unnecessary and delays wound healing. The intestine should be closely inspected for viability and damaged sections resected if appropriate (see p. 501). The abdominal cavity should be lavaged copiously with warmed, sterile saline. Open abdominal drainage (see p. 381) or suction drainage may be considered in animals with generalized peritonitis. Wound disruption after 10 to 21 days usually causes hernia formation rather than evisceration. Hernia repair in these animals may require excision of fibrotic tissues. Subsequent closure requires that tissue layers be accurately apposed. Healing may be delayed in debilitated, very young or very old, or hypoproteinemic animals; chromic gut suture should not be used for abdominal wall closure in these patients. Survival after major abdominal wall evisceration following ovariohysterectomy is high (Gower et al, 2009). The low mortality may be due to the fact that this event most commonly occurs in young, otherwise healthy animals and is promptly recognized and treated. Specific Diseases Abdominal hernias generally occur secondary to trauma, such as vehicular accidents or bite wounds; however, they occasionally occur as congenital lesions. Congenital cranial abdominal hernias (i.e., cranial to the umbilicus) have been reported in association with peritoneopericardial diaphragmatic hernias in dogs and cats. Abdominal hernias are false hernias because they do not contain a hernial sac. When associated with blunt trauma, they arise as a result of rupture of the wall from within caused by an increase in intraabdominal pressure while the abdominal muscles are contracted. The most common sites of traumatic abdominal hernias are the prepubic region and the flank. Cranial pubic ligament hernias often occur in association with pubic fractures (Fig. 19-4). Paracostal hernias may allow migration of abdominal contents along the thoracic wall (see Fig. 19-4). In rare cases, the abdominal contents enter the chest through defects in the intercostal muscles. Nearly half of the animals that suffer from traumatic abdominal hernia have serious concurrent injuries, including orthopedic (e.g., pelvis) and soft tissue injuries; thus a thorough physical examination of all affected animals should be performed. Umbilical hernias usually are congenital, caused by flawed embryogenesis (see Fig. 19-4). Umbilical vessels, the vitelline duct, and the stalk of the allantois pass through the umbilical ring in the fetus, but this aperture closes at birth, leaving an umbilical cicatrix. If the aperture fails to contract or is too large or is improperly formed, a hernia results. These hernias are lined by a peritoneal sac and are considered true hernias. The cause of umbilical hernias is seldom known, but most are thought to be inherited. Many male dogs with umbilical hernias are cryptorchid. Omphaloceles allow abdominal organs to protrude externally (eviscerate). The abdominal contents initially are covered by amniotic tissue, but this membrane covering is easily ruptured. Most affected neonates die or are euthanized at birth. If no concurrent abdominal injury or disease is identified, a variety of anesthetic protocols can be used to anesthetize the animal. See Table 19-2 for anesthetic management of animals that are in shock or are debilitated. See also subsequent chapters for detailed information about anesthetic management of patients with specific diseases (e.g., renal, pancreatic, hepatic). The abdominal wall is composed of four muscle layers (the external and internal abdominal oblique muscles, the rectus abdominis muscle, and the transversus abdominis muscle). Abdominal hernias may occur at insertions or attachments of these muscles or through muscle bellies themselves. In the dog, the cranial pubic ligament (prepubic tendon) is a band of transverse fibers that serves to attach the ventral abdominal muscles to the cranial border of the pubis. In cats, no distinct prepubic tendon exists (Beittenmiller et al, 2009); rather, the abdominal muscles attach directly to the pelvic brim (Fig. 19-5). As an alternative, suture the tendon remnant to the muscle fascia and periosteum covering the pubis, or anchor it to the pubis by drilling holes in the pubic bone through which sutures can be placed (Fig. 19-6). If the hernia extends into the femoral region, it may be necessary to suture the body wall to the medial fascia of the adductor muscles. When doing so, take care to avoid damaging the femoral vessels or nerves. A cranial sartorius muscle flap may be used to repair prepubic tendon injuries. Separate the cranial portion of the muscle from its caudal belly and the quadriceps femoris muscle. Then transect the cranial portion of the muscle at its distal insertion on the medial aspect of the patella and elevate it to the level of the proximal vascular pedicle (Fig. 19-7). Rotate the flap 180 degrees to the defect, and secure it using simple interrupted sutures. Use the flap to repair concurrent inguinal or femoral hernia, if present. Strong, absorbable suture (e.g., polydioxanone [PDS], polyglyconate [Maxon], poliglecaprone 25 [Monocryl], glycomer 631 [Biosyn]) or nonabsorbable suture (e.g., polybutester [Novafil], polypropylene [Prolene], nylon) should be used to repair abdominal or ventral hernias. Nonabsorbable suture materials may be preferred over absorbable suture materials because they maintain tensile strength for longer than a year (see p. 68); however, successful repair with absorbable suture materials has been reported. Marlex and Prolene synthetic mesh may be used to repair some large defects. A study of polypropylene mesh showed a substantial reduction in the diameter of the ductus deferens associated with an intense inflammatory reaction when the mesh was placed in contact with the spermatic cord of dogs (Goldenberg and Ferreira de Paula, 2005). Postoperative care of these patients is dictated by the presence of concurrent injury or disease. The patient should be kept quiet, and the wound should be checked frequently for infection or dehiscence. Vomiting, fever, and/or leukocytosis may indicate peritonitis (see p. 374). Beittenmiller, MR, Mann, FA, Constantinescu, GM, et al. Clinical anatomy and surgical repair of prepubic hernia in dogs and cats. J Am Anim Hosp Assoc. 2009;45:284. Goldenberg, A, Ferreira de Paula, J. Effects of the polypropylene mesh implanted through inquinotomy in the spermatic funiculus, epididium and testis of dogs. Acta Cirurgica Brasileria. 2005;20:461.
Surgery of the Abdominal Cavity
Preoperative Concerns
Anesthetic Considerations
![]() TABLE 19-1
TABLE 19-1



Antibiotics
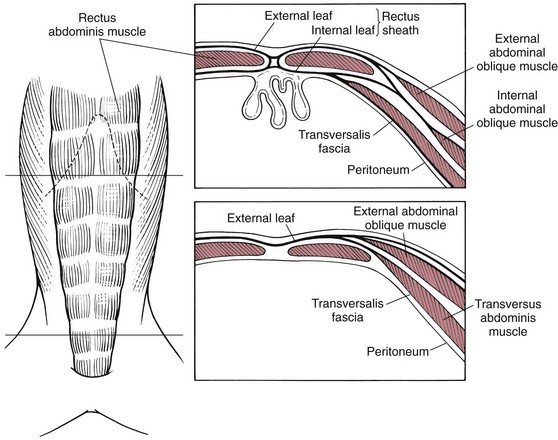
Surgical Anatomy
Surgical Techniques
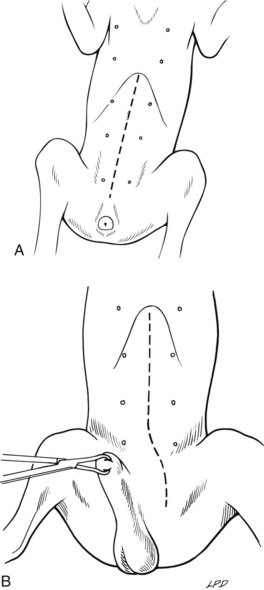
Ventral Midline Celiotomy in Cats and Female Dogs
Ventral Midline Celiotomy in Male Dogs
Abdominal Exploration
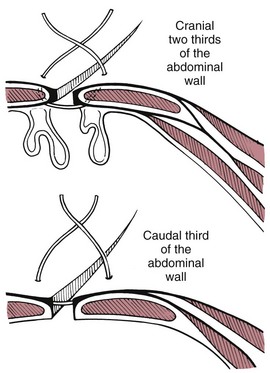
Abdominal Wall Closure
Postoperative Care and Assessment
Special Age Considerations
Umbilical and Abdominal Hernias
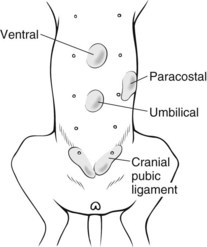
General Considerations and Clinically Relevant Pathophysiology
Surgical Treatment
Anesthesia
Surgical Anatomy
Surgical Techniques
Cranial pubic ligament (prepubic) hernias
Suture Materials and Special Instruments
Postoperative Care and Assessment
References
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Surgery of the Abdominal Cavity