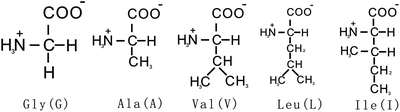
Fig. 7.1
General structure of an amino acid. Chemical structure of an amino acid showing the four different groups around the central α-carbon atom (with the exception of proline). The R group or side chain (red) attached to the α-carbon (blue) is different in each amino acid
All the 20 amino acids, except glycine (Gly or G), have four different groups arranged in tetrahedra around the central Cα atom which is thus known as an asymmetric center or chiral center and has the property of chirality. We note in Fig. 7.1 that the α-carbon is asymmetric, bonded to four different substituent groups: a carboxyl group, an amino group, an R group, and a hydrogen atom. Because of the tetrahedral arrangement of the bonding orbitals around the α-carbon atom of amino acids, the four different substituent groups can occupy two different arrangements in space, which are nonsuperimposable mirror images of each other. These two forms are called enantiomers or stereoisomers. Enantiomers are physically and chemically indistinguishable by most techniques, but can be distinguished on the basis of their different optical rotation of plane-polarized light. Molecules are classified as dextrorotatory (d; Greek dextro = right) or levorotatory (l; Greek levo = left) depending on whether they rotate the plane of plane-polarized light in a clockwise or an anticlockwise manner (Fig. 7.2). Surprisingly, only the l-amino acids are found in proteins, while d-isomers have been found only in small peptides of bacterial cell walls and in some peptide antibiotics.
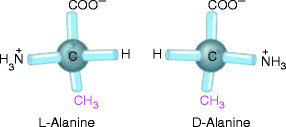

Fig. 7.2
Stereoisomerism in amino acids. The two stereoisomers of alanine, l– and d-alanine, are nonsuperimposable mirror images of each other (enantiomers)
Amino acids joined together by peptide bonds form the primary structure of a protein. The amino group of one molecule reacts with the carboxyl group of the other in a condensation reaction resulting in the elimination of water and the formation of a dipeptide (Fig. 7.3). A short sequence of amino acids is called a peptide, with the term polypeptide applied to longer chains of amino acids, usually of known sequence and length. When joined in a series of peptide bonds, amino acids are called “residues” to distinguish between the free form and the form found in protein. The peptide bond has a partial double-bond character.
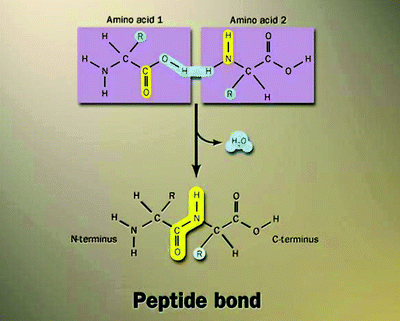

Fig. 7.3
Formation of a peptide bond. The peptide bond is a chemical, covalent bond formed between the α-amino group of one amino acid and the α-carboxyl group of another. Once two amino acids are joined together via a peptide bond to form a dipeptide, there is still a free amino group at one end and a free carboxyl group at the other, each of which can in turn be linked to further amino acids
7.2 Classification of Amino Acids
There are reasons to believe that amino acids are the oldest nutrients that have existed on earth. They have been used as the source of life and are sometimes called as the building blocks of life. There are as many as hundred thousand kinds of proteins that constitute the body, and these proteins are made with only 20 amino acids arranged in various combinations. The combination of the amino acids in proteins depends on the genetic code. In addition to the amino acids found in proteins, some amino acids not involved in the proteosynthesis are found in living organisms.
The names of the amino acids are often abbreviated, either to three letters or to a single letter. Thus, for example, alanine is abbreviated to Ala or A (Table. 7.1).
Table. 7.1
Names, symbols, chemical structures, and hydrophobicity indices of the 20 amino acids found in proteins
Name | Symbol | R group | Hydrophobicity | ||
|---|---|---|---|---|---|
3 Letter | 1 Letter | ||||
Aspartate | Asp | D | 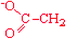 | 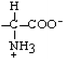 | −3.5 |
Glutamate | Glu | E | 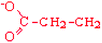 | 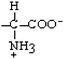 | −3.5 |
Lysine | Lys | K |  | 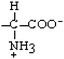 | −3.9 |
Arginine | Arg | R | 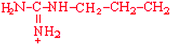 | 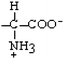 | −4.5 |
Histidine | His | H | 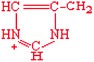 | 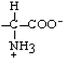 | −3.2 |
Tyrosine | Tyr | Y | 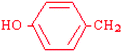 | 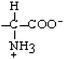 | −1.3 |
Tryptophan | Trp | W | 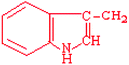 | 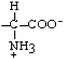 | −0.9 |
Phenylalanine | Phe | F | 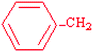 | 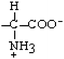 | 2.8 |
Cysteine | Cys | C | 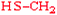 | 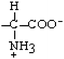 | 2.5 |
Methionine | Met | M | 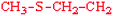 | 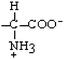 | 1.9 |
Serine | Ser | S | 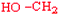 | 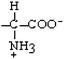 | −0.8 |
Threonine | Thr | T | 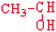 | 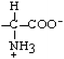 | −0.7 |
Asparagine | Asn | N | 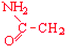 | 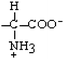 | −3.5 |
Glutamine | Gln | Q | 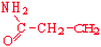 | 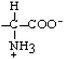 | −3.5 |
Glycine | Gly | G |  | 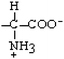 | −0.4 |
Alanine | Ala | A |  | 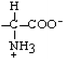 | 1.8 |
Valine | Val | V |  | 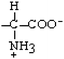 | 4.2 |
Leucine | Leu | L | 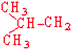 | 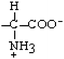 | 3.8 |
Isoleucine | Ile | I |  | 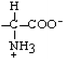 | 4.5 |
Proline | Pro | P | 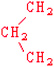 | 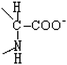 | −1.6 |
The hydrophobicity index informs on the relative hydrophobicity of amino acids. Higher positive value indicates stronger hydrophobicity. Hydrophilic amino acids have negative values. In a protein, hydrophobic amino acids are more likely to be located in the protein interior, whereas hydrophilic amino acids are more likely to face the aqueous environment.
7.2.1 Common Proteinogenic Amino Acids
Some of the 20 standard amino acids are called essential amino acids, because they cannot be synthesized by the body from other compounds through available metabolic pathways in the body, but instead must be provided by dietary sources. In humans, the nine essential amino acids are lysine, leucine, isoleucine, methionine, phenylalanine, threonine, tryptophan, valine, and (in children) histidine. It is thus necessary to provide these essential amino acids from proteins in food in well-balanced, appropriate amounts and, in some cases, to provide some individual amino acids which are limiting for protein biosynthesis. Some amino acids are considered as conditionally indispensable since in some physiological situation (for instance in neonates) and in some pathological situations, the needs for some amino acids are increased to such an extent that endogenous synthesis capacity is not able to provide enough amino acids related to these needs.
Based on the chemical structure of the R groups, the 20 amino acids of proteins can be divided into aliphatic amino acids, aromatic amino acids, and heterocyclic amino acids, and among them, aliphatic amino acids are the most.
Aliphatic Amino acids
1.
Neutral amino acids (Fig. 7.4):