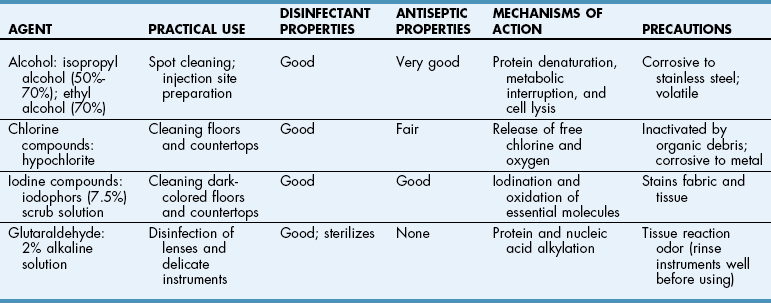
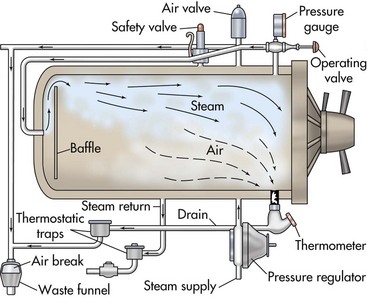
Chapter 2 Sterilization is the destruction of all microorganisms (bacteria, viruses, spores) on an item. It usually refers to objects (e.g., instruments, drapes, catheters, needles) that come in contact with tissue or enter the vascular system. Disinfection is the destruction of most pathogenic microorganisms on inanimate (nonliving) objects, whereas antisepsis is the destruction of most pathogenic microorganisms on animate (living) objects. Neither procedure claims to kill or inactivate all microorganisms, even when used properly. Antiseptics are used to kill microorganisms during patient skin preparation and surgical scrubbing (see Chapters 5 and 6); however, the skin is not sterilized. Cleaning is generally restricted in meaning to the physical removal of surface contaminants, usually with detergents or soap and water, ultrasound, or other methods. Although cleaning does remove soils and bacteria, it does not kill or inactivate viruses or bacteria. Disinfection usually involves the use of liquid compounds, such as phenol or its derivatives, alcohols, halides, aldehydes, quaternary ammonium compounds, chloroform, ethylene oxide (EtO), heavy metal ions, or dyes. Selection of the appropriate disinfectant depends on the desired result; some disinfectants are effective at destroying a limited number of microorganisms; others are effective at killing all organisms, including spores. Common disinfectants, their uses, and necessary precautions are listed in Table 2-1. Low-temperature, low-moisture processes, such as sterilization by EtO gas, hydrogen peroxide gas plasma, or peracetic acid, must be used for many medical devices (see pp. 13-15). Increasingly, operating room (OR) personnel are being asked to sterilize equipment more quickly and efficiently and at lower cost. Advanced sterilization systems that enable more rapid availability of wrapped, sterile devices and instruments may result in more rapid turnover of the OR suite and less “down time” between procedures. Swift and efficient sterilization of expensive heat- and moisture-sensitive medical and surgical devices (e.g., cameras, fiber-optic cables, rigid endoscopes) is particularly advantageous when costs of such equipment may limit their duplication in most veterinary practices. A low-temperature hydrogen peroxide gas plasma sterilization system that provides terminal sterilization of sophisticated instruments in 55 minutes is useful for such devices. Sterilization failure may occur if packs are wrapped too tightly or are improperly loaded in the autoclave or the gas sterilizer container. Instrument packs should be positioned vertically (i.e., on edge) and longitudinally in an autoclave. Heavy packs should be placed at the periphery, where steam enters the chamber. A small amount of air space is allowed between packs to facilitate the flow of steam (1 to 2 inches between the packs and away from the surrounding walls). Linen packs are loaded so that the fabric layers are oriented vertically (i.e., on edge). These packs are not stacked because increased thickness reduces penetration of the steam. Close supervision and exact standards for preparing, packaging, and loading of supplies are necessary for effective steam and gas sterilization. Sterilization indicators should be used (see p. 16). Gravity displacement sterilizer The most commonly used steam sterilizer in veterinary practice is the gravity (or “downward”) displacement sterilizer (Figs. 2-1 and 2-2). This sterilizer works on the principle that air is heavier than steam. Supplies to be sterilized are loaded into the inner chamber. A narrow, outer jacket-type chamber surrounds the inner chamber. Pressurized steam from the narrow, outer chamber enters the inner chamber and surrounds the supplies. Air in the inner chamber is pulled downward by gravity to the floor and exits through a temperature-sensitive valve. As steam accumulates and the temperature increases, the steam-release valve closes. Because the function of this sterilizer is based on the ability of air to move to the bottom of the autoclave, careful wrapping (see p. 3) and loading of supplies are critical (see previous discussion). The minimum time and temperature standards for a gravity displacement sterilizer are 10 to 25 minutes at 270° F to 275° F (132° C to 135° C) or 15 to 30 minutes at 250° F (121° C). Table 2-2 shows the recommended sterilization times for commonly sterilized items. Exposure Periods for Sterilization in Gravity Displacement Sterilizers Emergency or “flash” sterilization is performed when an unwrapped, nonsterile item must be sterilized quickly. A gravity displacement sterilizer is used for this purpose. The item is placed unwrapped in a perforated metal tray and is sterilized according to the manufacturer’s time and temperature recommendations. With detachable handles, sterilized items are transported to the OR in the metal tray. It is difficult to deliver flash-sterilized devices aseptically; the tray is hot, wet, and unwrapped, which means it collects dust, debris, and microorganisms more readily than dry, cool trays with biobarrier protection. This type of sterilization should be used only in emergencies when no alternative is available. The minimum time and temperature standard for a gravity flash sterilizer is 3 minutes at 270° F to 275° F (132° C to 135° C) for metal or nonporous items (i.e., items without a lumen) and 10 minutes at the same temperature for metal items with lumens, porous items (e.g., rubber, plastic), and autoclavable sterilized power tools. Flash sterilization generally is not recommended for implantable medical devices or power equipment unless specifically approved by the manufacturer. If an implant must be flashed, a “rapid read” biological spore test is used and can be read in 1 hour for a flash cycle. In flash sterilization, it is important to minimize the risk of contamination during transportation. The sterilizer should be located in the restricted area of the surgical suite or treatment site. It is advised to use rigid sterilization container systems (that are validated for use in flash sterilization; see Fig. 1-1 on p. 4) and the single-wrapper technique (if the sterile cycle is designed and labeled for this use) (AAMI, 2006; Carlo, 2007).
Sterilization and Disinfection
Disinfection
Sterilization
Steam Sterilization
Types of Steam Sterilizers
![]() TABLE 2-2
TABLE 2-2
ITEM
MINIMUM TIME REQUIRED, min, 250° F–254° F (121° C–123° C)
Scrub brushes (in dispensers, cans, individually wrapped)
30
Dressings (wrapped in muslin or paper)
30
Glassware (empty, inverted)
15
Instruments (wrapped in double-thickness muslin)
30
Instruments combined with suture, tubing, porous materials (wrapped in muslin or paper)
30
Metal instruments only (unwrapped)
15
Linen—maximum size 12 × 12 × 20 inches (6 kg wrapped)
30
Needles (individually packaged in glass vials or paper, lumens moist)
30
Needles (unwrapped, lumens moist)
15
Rubber catheters, drains, tubing (wrapped in muslin or paper, lumens moist)
30
Rubber catheters, drains, tubing (unwrapped, lumens moist)
20
Utensils (wrapped in muslin or paper, on edge)
20
Utensils (unwrapped, on edge)
15
Syringes (unassembled, individually packaged in muslin or paper)
30
Syringes (unassembled, unwrapped)
15
Suture—silk, cotton, nylon (wrapped in paper or muslin)
30
Solutions: 75 to 250 ml
20 (slow exhaust)
500 to 1000 ml
30 (slow exhaust)
1500 to 2000 ml
40 (slow exhaust)
Flash sterilizer
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Sterilization and Disinfection