Staphylococci represent a diverse genus of facultatively anaerobic bacteria that belong to the family Micrococcaceae. They are gram-positive, catalase-positive cocci that tend to occur in clusters. They are widely distributed among animals and are common commensals of the skin and mucous membranes. Staphylococci are classic opportunistic pathogens, being found in or on a high percentage of healthy individuals, while also being an important cause of disease. Staphylococcal species are divided into two main groups based on the production of the enzyme coagulase. Coagulase-positive staphylococci are the most virulent group and are most often associated with disease. The most important coagulase-positive species in dogs and cats are Staphylococcus pseudintermedius, Staphylococcus aureus, and Staphylococcus schleiferi ssp. coagulans. Staphylococcus intermedius was previously considered the most important Staphylococcus in dogs and cats, but it is now known that what was previously identified as S. intermedius is truly the closely related species S. pseudintermedius.53,201 S. intermedius is actually rare to nonexistent in dogs and cats. Previously published reports of S. intermedius almost certainly actually involved S. pseudintermedius, and for consistency and accuracy, these will be referred to as S. pseudintermedius throughout this chapter. There are differences in the epidemiology of staphylococcal colonization and infection between different staphylococci and between dogs and cats. Staphylococci have often been referred to as host-adapted species, but it is clear that dogs and cats can be infected by many different staphylococcal species, and it has been suggested that the “one-pathogen–one host” concept should no longer be accepted.156 Regardless, there are certainly differences in the predominant staphylococcal species between animal species, but there is also extensive overlap, and most staphylococci can be found in multiple species, albeit at varying prevalences. S. pseudintermedius is a true canine commensal and studies have reported colonization rates of 31% to 68% in healthy dogs.69,69a,90,97,99 Higher rates (up to 100%) have been reported in puppies.198 It is possible, if not likely, that colonization rates in adult dogs could be higher if multiple sites and enrichment culture methods were used, and it is reasonable to assume that a high percentage of healthy dogs are carrying S. pseudintermedius at some body site at any given time. Colonization starts early in life, with puppies acquiring S. pseudintermedius from the dam within 8 hours after birth.198 Various body sites can be colonized, particularly the nasal passages, oral cavity, skin and perineal mucosa.69,99 Duration and dynamics of colonization are not known. It is likely that some dogs are colonized for prolonged periods of time, if not lifelong, whereas for others colonization is transient and recurrent. Only a small percentage of dogs develop a clinical infection, despite widespread exposure, and infections typically require some form of underlying cause such as skin trauma. S. pseudintermedius is less prevalent in cats, being reported in 6.8% to 22% of healthy cats.2,97,132,133 As with dogs, opportunistic infections can develop, but the influence of long-term or transient colonization on risk of disease is unknown. In parallel with colonization rates, S. pseudintermedius infections are less common in cats compared to dogs.156 Methicillin-resistant (MR) S. pseudintermedius (MRSP) has emerged and disseminated widely in each successive year.* It is considered a serious emerging problem in small animal veterinary medicine and one that requires urgent action to control its spread.91 As with methicillin-susceptible strains, MRSP can be found in or on clinically healthy dogs and cats. Reported prevalences of colonization range from 1.5% to 17% in dogs, and 1.2% in healthy cats.60,90,97,98,239 As with methicillin-susceptible staphylococci, MRSP is an opportunistic pathogen, and colonization does not necessarily lead to disease. It is unclear whether colonization is an actual risk factor for subsequent development of infection, but it is reasonable to assume that colonization does pose some degree of risk to an animal, albeit presumably rather low. Risk factors for MRSP colonization have not been adequately investigated, but if extrapolation of information from MR S. aureus (MRSA) is reasonable, prior antimicrobial exposure is likely to be a risk factor. MRSP infections are not distinguishable clinically from those caused by susceptible strains, but are increasingly problematic because of the limited treatment options. Limited study of risk factors for infection has been performed but antimicrobial administration, hospitalization, or surgery within 30 days before the onset of infection were associated with MRSP versus methicillin-susceptible S. pseudintermedius infection in one study.249 S. aureus can be isolated from up to 12% to 14% of clinically healthy dogs and 4.3% to 20% of clinically healthy cats,1a,2,90,97,192a but it may not truly be a commensal organism. The highest isolation rates are from skin and ears.64 It is possible that isolation of S. aureus represents transient colonization or contamination acquired from humans. This is probably particularly true with dogs. A study of dogs and their owners reported that in 50% of households where S. aureus was isolated from both a dog and family member, the S. aureus strains were indistinguishable, strongly suggesting interspecies transmission.97 The situation with cats is less clear. Given the higher apparent prevalence of S. aureus in cats in some studies and the lack of another predominant coagulase-positive Staphylococcus sp. commensal in cats, it is possible that S. aureus is a true feline commensal. Regardless, this is perhaps a moot point in terms of the role of S. aureus in disease. It can clearly be isolated from a small but still appreciable percentage of clinically healthy dogs and cats and can also cause disease in certain situations. Risk factors for colonization have not been adequately investigated and may relate to the amount or degree of human contact. Much of the recent attention regarding S. aureus in dogs and cats has been focused on MRSA, both in terms of animal health and zoonotic disease. The emergence of MRSA in dogs and cats appears to be a direct reflection of MRSA in the human population: MRSA infections in pets were noted after MRSA emerged as an important cause of disease in the general human population and with MRSA strains from animals usually reflecting predominant human strains.* Currently, MRSA can be isolated from 0% to 3.3% of clinically healthy dogs and 0% to 4% of clinically healthy cats.† Higher rates can be encountered periodically in veterinary facilities 116a,139,159b,248; however, most instances of MRSA isolation are not associated with veterinary hospitals. Ownership by a human health care worker and participation in hospital visitation programs have been identified as risk factors for MRSA colonization in dogs, and are logical based on the increased likelihood of exposure to colonized people.31,130 Contact with children has also been identified as a risk factor.130 Although these, and potentially other, risk factors should be considered, MRSA can be identified in any animal, and absence of known risk factors should not lead to excluding MRSA from consideration. As with methicillin-susceptible S. aureus (MSSA), colonized animals typically have no signs of infection and may never develop a clinical illness. In humans and horses, MRSA colonization is known to be a risk factor for clinical MRSA infection in certain circumstances (e.g., after admission to the hospital).111,112 It is reasonable to assume that this also applies to dogs and cats, yet this is not proven. Although MRSA colonization has zoonotic concerns (see the later section on Public Health Considerations), the animal health implications of MRSA colonization in clinically healthy animals are likely minimal. This species tends to be less commonly identified than S. pseudintermedius and S. aureus but can be found in 0.8% to 4% of dogs and 0% to 2% of cats.2,90,90 Colonization with methicillin-resistant S. schleiferi ssp. schleiferi (MRSS) has been identified in 0% to 2% of dogs,90,97,97 and MRSS infections are being increasingly reported.117,119,119 CoNS are commonly found in healthy dogs and cats, along with virtually all other mammalian species. In dogs, CoNS are commonly isolated from the skin, nasal and oral cavities, pharynx, perineal mucosa, gastrointestinal tract, and conjunctiva. S. xylosus, S. epidermidis, and S. sciuri are commonly isolated from clinically healthy dogs,4,46,46 but various other species can be found. The prevalence of colonization is high,220 and with adequate effort CoNS could probably be found at one or more sites from most (if not all) dogs. Colonization with CoNS is also common in clinically healthy cats, with high isolation rates from skin, saliva, and the vagina.42,132,132 S. felis appears to be most common, with Staphylococcus haemolyticus, S. epidermidis, Staphylococcus simulans, and Staphylococcus saprophyticus also isolated.32,133 The preferred site of residence of different CoNS varies, with some found in many locations and others only in specific body sites. Despite widespread colonization, CoNS infections are uncommon. In human medicine, CoNS are primarily a concern in hospitalized individuals, with virtually all S. epidermidis infections originating in hospitals.11 Community-associated CoNS infections in humans are usually UTIs caused by S. saprophyticus.11 The situation may be similar in dogs and cats, with most CoNS being of minimal concern but with some species (i.e., S. schleiferi ssp. schleiferi, and S. felis, as described later) being potentially important causes of community-onset disease. Occasional synergistic pathogenicity has been reported with CoNS and other organisms.22a One limitation to our understanding of differential pathogenicity of CoNS species is the typical lack of speciation. Usually, CoNS are reported as CoNS, not an individual CoNS species. Therefore, identification of patterns involving individual species is more difficult. Methicillin resistance is not uncommon in commensal CoNS. Studies have reported prevalences ranging from 5% to 13% in clinically healthy dogs, and of 5% in clinically cats.14,16,16 MR strains are no more pathogenic than methicillin-susceptible strains, and the implications of colonization with MR-CoNS are typically inconsequential. Staphylococci can possess a wide array of potential virulence factors; however, the role that many of these play in the actual development of disease is variable. Virulence factors can be considered on the basis of different general properties. Some virulence factors facilitate adhesion to and colonization of host tissues. Others encode secreted enzymes and toxins that are responsible for invasion as well as local and distant disease. The pathophysiology of disease is complex and only superficially understood. Interactions between various virulence factors are presumably critical, because no single virulence factor has been shown to be sufficient to establish an infection.56 The ability to colonize body sites is an important component of virulence, because it allows staphylococci to remain on or in the body waiting for an opportunity to cause an infection, particularly after an underlying insult such as a break in the body’s physical or immunological barriers. A great number of surface adhesins have been identified. Adhesins do not directly result in damage to host tissues, but create an environment where staphylococci can persist with the potential for development of infection at any site in the body due to other host and bacterial factors. Adhesins may also facilitate evasion of the immune system. For S. aureus, the most important is probably protein A, which binds to the Fc portion of IgG.154 Other important surface proteins including clumping factors, fibronectin binding proteins, coagulase, and collagen binding protein. Less is known about S. pseudintermedius, but evidence indicates that this species expresses surface proteins that are similar to those from S. aureus.79 Various secreted enzymes facilitate development and progression of local disease through degradation of host tissues. This further compromises physical barriers and helps the bacterium use those tissues as a nutrient source. Similarly, staphylococci can produce a range of toxins that can potentiate local infection and tissue injury. Various hemolysins can lyse erythrocytes and other body cells. Proteases can cleave antibodies, play a role in protection against neutrophil defensins and platelet microbicidal peptides, contribute to destruction of tissue protein, and potentiate invasion.103 Hyaluronidase and hyaluronate lysate may contribute to virulence through digestion of hyaluronic acid in connective tissue and promoting degradation of tissues.103 Lipase has a negative effect on the host’s immune function and may help the bacterium harvest nutrients from the local environment.103 Alpha toxin is a potent membrane-damaging toxin that can produce local cell damage. Leukocidins damage leukocytes and lipid membranes. One, the Panton Valentine leukocidin, has received much attention because of its potential role in severe MRSA skin and soft tissue infections, as well as necrotizing fasciitis and necrotizing pneumonia in humans.74,120,150,241 This leukocidin has been found in MRSA from dogs and cats,31,187,237,240 but its role in disease is still unclear. A similar leukocidin has also been reported in S. pseudintermedius,184 but its clinical relevance is also unknown. An exfoliative toxin gene was found in higher prevalence in S. pseudintermedius isolates from dogs with superficial pyoderma (23.2%) as compared to those with clinically healthy skin (6.1%).116b Most of these virulence factors are involved with disease at the site of clinically evident infection, such as with a wound infection or pyoderma. In some situations, staphylococci can produce “distant” diseases through the production of selected toxins. A well-characterized example of this in humans is food poisoning through ingestion of preformed enterotoxins produced in improperly stored food.118,158 Enterotoxin production is mediated by several staphylococcal enterotoxin genes, many of which can be found in S. aureus and S. pseudintermedius isolates from dogs.4,20,58,108 Staphylococcal enterotoxin-mediated disease is not known to occur in dogs and cats, but there is no reason to believe that pets are inherently resistant to the effects of staphylococcal enterotoxins. Rather, there is likely less common exposure to contaminated foodstuffs and less consideration of staphylococcal food poisoning when examining dogs and cats with diarrhea. Other toxins can produce distant diseases in humans, although as with enterotoxins, their role in disease in dogs and cats is poorly characterized. In S. aureus, these toxins include exfoliative toxins A and B, which cause staphylococcal scalded skin syndrome, and toxic shock syndrome toxins, which cause toxic shock syndrome.154 S. intermedius exfoliative toxin can be found in S. pseudintermedius isolates from a large percentage of dogs and cats,62,63,127,225 and it may play a role in pyoderma and otitis, but that has not yet been proven. Another potentially important aspect of virulence is the ability to form biofilms. Biofilms are extracellular polysaccharide networks that help bacteria escape the effects of antimicrobials and the immune system. Biofilm may be most relevant for infections of implants and invasive devices (e.g., intravenous catheters). Biofilm formation has been demonstrated for various staphylococci, including S. pseudintermedius and S. aureus.5,75 Although not a specific virulence factor, the ability of staphylococci to acquire resistance to antimicrobials can play a major role in disease. Staphylococci have an impressive ability to acquire resistance factors and to mutate to become resistant during treatment. The concerns about antimicrobial resistance have been heightened by the emergence of methicillin-resistant staphylococci (MRS).250 MRS are resistant to all β-lactams (penicillins, cephalosporins, carbapenems) through production of an altered penicillin-binding protein. This is mediated by the mecA gene, a gene that is located on a staphylococcal chromosomal cassette (SCCmec).250 This site also has the ability to acquire other resistance genes. Although methicillin resistance only involves β-lactam resistance, and some MRS are only resistant to β-lactams, many MRS are resistant to a wide range of antimicrobials. MRSA and MRSP are emerging as serious problems in veterinary medicine. Some strains, particularly MRSP strains, are now resistant to almost all available treatment options, greatly complicating management of clinical illness in which they are involved.91 Methicillin resistance is also commonly present in CoNS; however, this is of less clinical concern because, whether resistant or not, CoNS are less pathogenic than coagulase-positive species. Methicillin resistance does not need to be present for staphylococci to be resistant to other antimicrobials; however, multidrug-resistant but methicillin-susceptible staphylococci appear to be relatively uncommon. Staphylococci can cause a wide range of opportunistic infections, ranging from mild pyoderma to rapidly fatal necrotizing infections. Any body system can be affected, but skin and soft tissue infections are most common. Severe manifestations of staphylococcal infection include staphylococcal toxic shock syndrome, septicemia, and necrotizing fasciitis (see also Streptococcal Infections, in Chapter 33).22a,250 S. pseudintermedius accounts for the vast majority of cases of pyoderma in dogs, and a lesser percentage in cats. It also causes a range of other infections including wound infections, surgical site infections, septic arthritis, osteomyelitis, UTIs, endocarditis, liver abscess, peritonitis, and ocular infections.* The severity of disease is highly variable. Serious, including fatal, invasive infections can occur but are uncommon. Fatal toxic shock and cellulitis associated with S. pseudintermedius has been reported in one dog.81 Necrotizing fasciitis, a rare but devastating disease, is most commonly associated with Streptococcus canis, but S. pseudintermedius has also been implicated.250 MRSP causes the same spectrum of disease, with a predominance of skin, ear and other soft tissue infections.247,249 Postoperative infections, particularly involving tibial plateau leveling osteotomy surgery, appear to be increasingly common.247,249 Other infections can also occur with susceptible strains. There is currently no indication that MRSP infections are more serious than infections caused by methicillin-susceptible strains, although limitations in treatment of the former could result in greater likelihood of a poorer outcome. A similar range of infections can be produced by S. aureus. The main difference between S. pseudintermedius and S. aureus is the lower prevalence of disease with the latter, not disease location or severity. S. aureus infections may be more common in cats compared to dogs. Clinically, MRSA infections are indistinguishable from those caused by MSSA, producing predominantly skin and ear infections, and lesser numbers of other opportunistic infections.† Diskospondylitis was caused by MRSA in two dogs.204a A greater risk of MRSA infection of dogs was found in dogs receiving antimicrobial drugs, especially quinolones. It is possible that infections more commonly associated with human contact (i.e., wound and surgical site infections contaminated by hands of owners or veterinary personnel) are more likely to be caused by S. aureus (including MRSA), but objective data are lacking. The most common clinical manifestations of infection are pyoderma and otitis externa, but infections of other sites such as the genitourinary tract and respiratory tract can occur.‡ This is clinically indistinguishable from disease caused by other staphylococci, although it has been suggested that this species may tend to produce more superficial skin disease compared to S. pseudintermedius and S. aureus.156 The role of this species in disease may be underestimated because of the failure of many diagnostic laboratories to differentiate it from S. pseudintermedius. Although CoNS are generally considered as a group, it is possible that there are differences between CoNS species. This mainly relates to two organisms: S. schleiferi ssp. schleiferi and S. felis. S. schleiferi ssp. schleiferi has been implicated as a cause of pyoderma and otitis.72,148,148 A primary role of S. felis in UTIs in cats has also been suggested based on a study that found this species in 19.8% of cats with UTIs.135 These two species may, therefore, be primary pathogens in community-associated disease, unlike most other CoNS. Antimicrobial susceptibility testing is a critical component of diagnostic testing. Given the variability in susceptibility patterns among staphylococci and the emergence of multidrug-resistant strains like MRSP and MRSA, it is important that proper susceptibility testing be performed to guide treatment. Veterinarians should ensure that the diagnostic laboratory they use followed standard Clinical and Laboratory Standards Institute (CLSI) guidelines for performing and interpreting susceptibility testing results, because otherwise, erroneous and misleading results can be obtained. In general, methods to detect antimicrobial susceptibility among staphylococci are well developed and validated. Problems with identification of methicillin resistance in S. pseudintermedius have been identified, with evidence that oxacillin and cefoxitin breakpoints that have been used to determine susceptibility or resistance will misidentify many MRSP as susceptible.21,170,171,247 Changes in CLSI guidelines for susceptibility testing of S. pseudintermedius have decreased the breakpoints used to determine susceptibility and will, it is hoped, rectify this situation. Another area of concern is determination of clindamycin resistance because of inducible resistance, as is discussed under Systemic Antimicrobials. There are no other viable diagnostic assays. Molecular diagnostic methods are becoming more popular for various pathogens. However, despite the presence of commercially available testing, there are no validated molecular tests for diagnosis of staphylococcal infections in dogs and cats. Although polymerase chain reaction tests offer the potential for a rapid result, adequate test development and validation is required to ensure sensitivity and specificity. Animal species-specific validation is required. There are commercial assays for rapid detection of MRSA in humans; however, these have not been validated in animals, and investigation of one test in horses demonstrated poor performance.10 Therefore, any tests for dogs and cats must be proven to be effective in those species. Broad-range real-time polymerase chain reaction is becoming more commonly investigated as a potential diagnostic tool. This type of testing uses primers to detect all known bacteria and could offer a rapid test for bacterial infections. However, these have not been validated in animals. Further, they are not as useful in infections of nonsterile sites or where mixed infections may be present, thereby limiting the relevance for most staphylococcal diseases in dogs and cats. Although culture has disadvantages including slow turnaround time, it should still be considered the clinical standard. Treatment is ideally based on in vitro susceptibility testing. This is particularly true as the prevalence of multidrug-resistant staphylococci increases. Broad recommendations for treatment of staphylococcal infections are difficult to make because of the variability in antimicrobial resistance rates. The prevalence of resistance to various antimicrobials varies between (and even within) regions, so knowledge of local susceptibility patterns is useful (Web Table 34-1). Although published data on susceptibility patterns such as those presented here can provide some useful information, the limitations of such data must be acknowledged. With geographic variations in resistance rates, published data from other areas may not reflect a clinician’s true population. Published data may overestimate resistance if they are based on referral cases or when cultures are primarily submitted from cases that have not responded to initial therapy. Conversely, there can be relatively rapid changes in susceptibility trends, particularly because MRS dissemination, and historic data, even those from a few years earlier, may not be representative of the current situation (Web Tables 34-2 and 34-3). Knowledge of resistance trends in the practice is a more useful to guide empiric therapy and treatment while awaiting culture results, but nothing takes the place of culture and susceptibility data from the actual patient. WEB TABLE 34-1 Antimicrobial Susceptibility of Staphylococcus pseudintermedius (% Susceptible) NR, Not reported. WEB TABLE 34-2 Susceptibility Data for Methicillin-Resistant Staphylococcus pseudintermedius (% Susceptible) NR, Not reported. WEB TABLE 34-3 Susceptibility of Methicillin-Resistant Staphylococcus aureus from Dogs and Cats (% Susceptible) In general, resistance is uncommon to first-generation cephalosporins (e.g., cephalexin) or β-lactamase resistant penicillins or penicillin/β-lactamase inhibitor combinations (e.g., amoxicillin-clavulanate), and these drugs should typically constitute the first line of treatment of staphylococcal infections.92,180a,180b In cases where first-line choices are not useful, there are various other drugs, such as cephalosporins or doxycycline, that can be used with success, depending on the susceptibility of the strain. Aminoglycosides can also be effective, although the parenteral route of administration can be problematic. The role of quinolones in the treatment of staphylococcal infections is somewhat controversial. Given their in vitro activity, ability to penetrate the skin, and oral form of administration, quinolones have been popular choices for staphylococcal infections. Clinical efficacy has been reported for the treatment of pyoderma with quinolones, albeit typically in uncontrolled trials,114,172,172 and quinolones are used as a first-line therapy by some clinicians. However, concerns about quinolone resistance, in terms of both clinical treatment failure and public health consequences, have led to recommendations that quinolones be used only when resistance to other antimicrobials is present or likely.92 Prudent use of quinolones may be especially important with MRSA. In humans, quinolones are considered to be contraindicated for the treatment of MRSA infections because of poor clinical response and rapid development of resistance.85 This has not been objectively investigated in dogs and cats, but there is no reason to suspect that it would be different in these species, so quinolones probably should be avoided whenever possible as treatments of resistant staphylococcal infections such as MRSA and MRSP. There has been less discussion about their indication in methicillin-susceptible infections. These should perhaps be approached with caution as well, because the presence or absence of methicillin resistance should be of limited relevance to the likelihood of treatment failure or emergence of resistance with quinolone treatment. It is reasonable, therefore, to recommend prudent use of this drug class for any staphylococcal infection and to avoid quinolones in any serious or life-threatening staphylococcal infection if possible. Clindamycin may be an option in some cases, including as a first-line treatment for superficial pyoderma,92 but resistance is a concern and is not always readily apparent with routine testing.63 Inducible clindamycin resistance can be present, particularly in S. aureus.63 With this phenomenon, isolates appear to be susceptible to clindamycin in vitro; however, resistance is induced on exposure in vivo, and treatment failure is expected. One study of reported inducible resistance in 71% of MRSA strains from animals,190 whereas another study reported that 55% of erythromycin-resistant, clindamycin-susceptible MRSA from dogs were inducibly resistant.65 Inducible resistance can be detected in vitro using a “D-test”; however, this is not widely available in veterinary diagnostic laboratories. S. aureus isolates that are reported as erythromycin resistant but clindamycin susceptible should be considered resistant to clindamycin unless a D-test has indicated the absence of inducible resistance. Inducible resistance appears to be rare in S. pseudintermedius,65 but because it has been reported,29,63 it would be prudent to avoid clindamycin for the treatment of serious infections caused by erythromycin-resistant strains without demonstration of absence of inducible clindamycin resistance. Trimethoprim-sulfonamide is commonly effective against MRS in vitro. Safety concerns are significant with this drug combination and include keratoconjunctivitis sicca, arthropathy, and other idiosyncratic effects (see the Drug Formulary in the Appendix). Trimethoprim-sulfonamide can be used safely in most patients and is appropriate in situations with few other viable options. It should probably be avoided in animals at high risk for keratoconjunctivitis sicca, as well as Doberman pinschers because of the apparent high risk for idiosyncratic arthropathy. Tylosin has been used in the past for treatment of staphylococcal skin infections in dogs. A majority (82.6%) of the strains of S. pseudintermedius isolated from dogs with pyoderma were found to be susceptible to tylosin in vitro.207a These were from dogs that had not received antibacterial therapy for at least 3 months prior to sample submission. The emergence of MRSP, MRSA, and other MRS has complicated treatment. Highly resistant strains can be present, including some with few treatment options. This seems to be more common with MRSP, and some MRSP isolates that are resistant to almost all available drugs are being encountered in some regions. This has led to the consideration for use of antimicrobials that are critically important in human medicine, such as vancomycin.246 Vancomycin, linezolid, tigecycline, and quinupristin-dalfopristin are drugs that are important for treatment of serious multidrug-resistant infections in humans. Their use in animals raises many ethical questions that are difficult to answer. Concerns include increased pressure toward resistance of animal-harbored staphylococci and their subsequent transmission to humans. The concerns of public health risk are often opposed with concerns about animal health and welfare if proper treatment is not provided, and the fact that animal use makes up a minuscule percentage of overall use of these drugs. There is currently no consensus on the topic. At a minimum, these drugs should be reserved for situations where there are no other viable treatment alternatives; where the infection is not amenable to other (i.e., local) therapy; and where the infection is life threatening but potentially treatable. Before considering drugs such as these, all other therapeutic options should be considered. Currently, it is rare to require drugs such as these because there is almost always another treatment option. As is common in veterinary medicine, there is limited objective information to guide duration of therapy. This depends more on the type and location of infection than on the Staphylococcus species that is involved. There is no evidence that infections caused by MRS need longer or otherwise different treatment compared to infections caused by susceptible strains once appropriate antimicrobial therapy is started. Commonly used dosages for treatment of staphylococcal infections are listed in Table 34-1 and the Drug Formulary (see the Appendix) and also under skin infections, Table 84-1. TABLE 34-1 Drug Therapy for Staphylococcal Infections in Dogs and Cats
Staphylococcal Infections
Etiology
Epidemiology
Staphylococcus pseudintermedius
Staphylococcus aureus
Staphylococcus schleiferi ssp. coagulans
Coagulase-Negative Staphylococci
Pathogenesis
Clinical Findings
Staphylococcus pseudintermedius
Staphylococcus aureus
Staphylococcus schleiferi ssp. coagulans
Coagulase-Negative Staphylococci
Diagnosis
Therapy
Systemic Antimicrobials
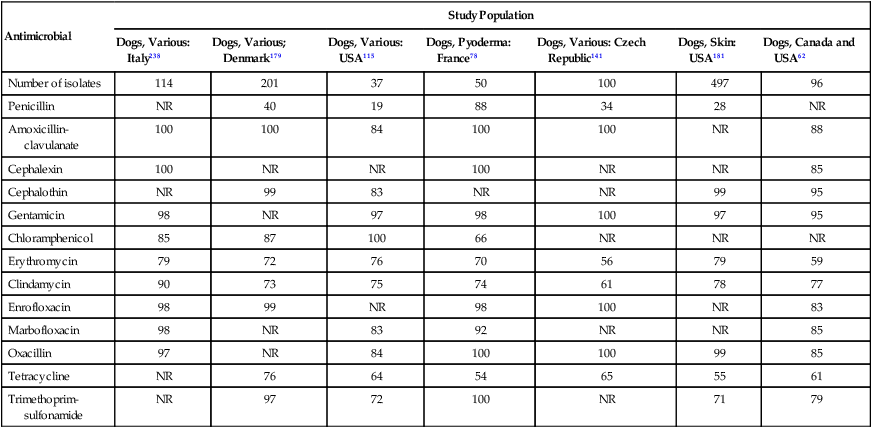
Antimicrobial
Study Population
Dogs, Various: Italy238
Dogs, Various; Denmark179
Dogs, Various: USA115
Dogs, Pyoderma: France78
Dogs, Various: Czech Republic141
Dogs, Skin: USA181
Dogs, Canada and USA62
Number of isolates
114
201
37
50
100
497
96
Penicillin
NR
40
19
88
34
28
NR
Amoxicillin-clavulanate
100
100
84
100
100
NR
88
Cephalexin
100
NR
NR
100
NR
NR
85
Cephalothin
NR
99
83
NR
NR
99
95
Gentamicin
98
NR
97
98
100
97
95
Chloramphenicol
85
87
100
66
NR
NR
NR
Erythromycin
79
72
76
70
56
79
59
Clindamycin
90
73
75
74
61
78
77
Enrofloxacin
98
99
NR
98
100
NR
83
Marbofloxacin
98
NR
83
92
NR
NR
85
Oxacillin
97
NR
84
100
100
99
85
Tetracycline
NR
76
64
54
65
55
61
Trimethoprim-sulfonamide
NR
97
72
100
NR
71
79
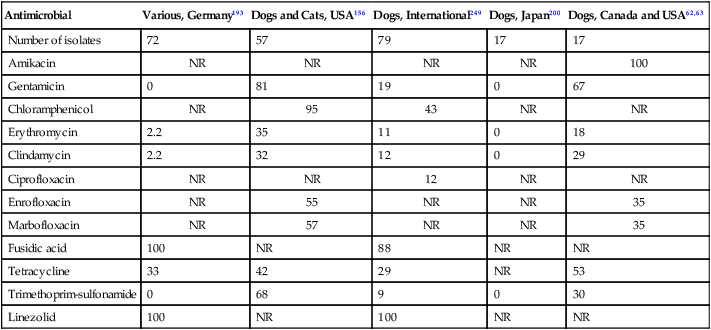
Antimicrobial
Various, Germany193
Dogs and Cats, USA156
Dogs, International249
Dogs, Japan200
Dogs, Canada and USA62,63
Number of isolates
72
57
79
17
17
Amikacin
NR
NR
NR
NR
100
Gentamicin
0
81
19
0
67
Chloramphenicol
NR
95
43
NR
NR
Erythromycin
2.2
35
11
0
18
Clindamycin
2.2
32
12
0
29
Ciprofloxacin
NR
NR
12
NR
NR
Enrofloxacin
NR
55
NR
NR
35
Marbofloxacin
NR
57
NR
NR
35
Fusidic acid
100
NR
88
NR
NR
Tetracycline
33
42
29
NR
53
Trimethoprim-sulfonamide
0
68
9
0
30
Linezolid
100
NR
100
NR
NR
Cats, USA155
Dogs and Cats, USA156
Number of isolates
13
39
Gentamicin
92
92
Chloramphenicol
77
90
Erythromycin
0
15
Clindamycin
0
28
Enrofloxacin
0
10
Marbofloxacin
0
10
Tetracycline
69
64
Trimethoprim-sulfonamide
92
97
Druga
Species
Doseb (mg/kg)
Route
Interval (hours)
Duration (days)
Amoxicillin-clavulanate
B
12.5–25
PO
12
prn
Cephalexin or cefadroxil
D
22
PO
12
prn
Cephalexin
C
22–30
PO
12
prn
Cefadroxil
C
22
PO
24
prn
Clindamycinc
B
11
PO
12
14–42
Quinolonesd
B
Variesc
PO
24
prn
Erythromycin
D
10–20
PO
8
prn
Chloramphenicol
D
25–50
PO, SC, IM, IV
8
prn
C
10–20
PO, SC, IV
12
prn
Trimethoprim-sulfonamide
D
22
PO
12
prn ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Staphylococcal Infections
