Chapter 5 SPERM PHYSIOLOGY
SPERMATOGENESIS
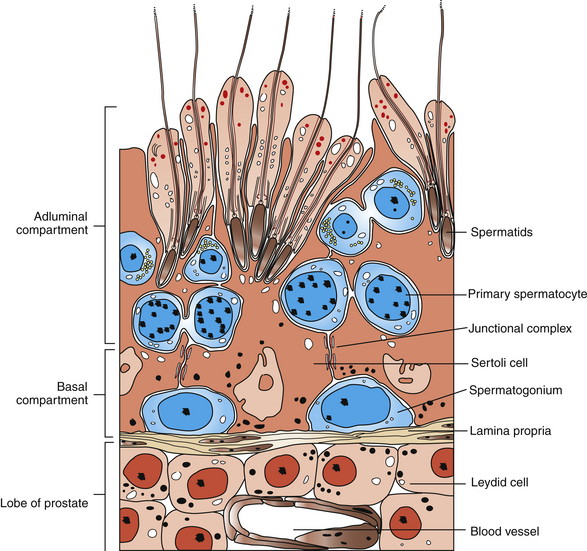
Spermatogenesis is the process of sperm production by the seminiferous epithelium during which spermatogonial stem cells generate spermatocytes, which differentiate, multiply, and generate spermatozoa. This occurs within the seminiferous tubules, which are the major constituent of the testicular parenchyma in the stallion’s testis. Seminiferous tubules comprise a myoid cell layer surrounding the basal lamina, which surrounds a complex of somatic and germ cells. The Sertoli cell is a somatic cell that serves a structurally and physiologically supportive role to each successive generation of germ cells. Each sperm cell and its precursors are physically embedded between or in contact with the Sertoli cells such that each Sertoli cell contacts a large number of germ cells at various stages of development. Sertoli cells are spaced along the tubule, and specialized tight junctional complexes between neighboring Sertoli cells form the epithelial portion of the blood-testis barrier. This provides isolation of the diploid spermatogonia and preleptotene spermatocytes from subsequent haploid postmeiotic spermatocyte cell types, spermatids, and the released spermatozoa.1 The final product of spermatogenesis, the spermatozoon, is the last of numerous successive cellular generations that result from a repeatable sequence of developmental events within the tubule. The culmination of the process is the release from Sertoli cells of spermatids into the lumen of the convoluted portion of the seminiferous tubules and subsequent passage of the spermatozoa into the straight portion of the tubules, tubuli efferentes, and caput epididymis.
Spermatogenesis consists of three developmental phases: (1) spermatocytogenesis (mitotic divisions of spermatogonia), (2) meiosis, and (3) spermiogenesis (maturation and differentiation of spermatids). Amann9 indicated that the three phases correspond to durations of approximately 19.4, 19.4, and 18.6 days, respectively, leading to a total duration of spermatogenesis in stallions of approximately 57–58 days.
Beginning with spermatocytogenesis, the Al-type spermatogonia divide mitotically to (1) produce daughter cells that are committed to completion of spermatogenesis and (2) repopulate the uncommitted stem cell population (Ao) to maintain a progenitor population capable of continuing the spermatogenic cell lineage. The latter is localized to the seminiferous epithelium nearest the basement membrane (Fig. 5-1). The Al spermatogonia mitotically divide at regular intervals to enter spermatogenic cell development (approximately every 12.2 days), differentiating into at least five subtypes (Al-3, Bl, B2) prior to the appearance of preleptotene primary spermatocytes,3 which undergo the first meiotic division. The spherical secondary spermatocytes resulting from completion of meiosis then undergo morphologic differentiation. The resulting spermatids have a characteristic elongated cellular shape, condensed nucleus, and axoneme for motility. Each generation of spermatogonia and spermatocytes is intimately linked by intercytoplasmic bridges and undergoes synchronous cellular division and differentiation. Consequently, the cells advance toward the seminiferous epithelial lumen and away from the basement membrane with each successive cell division. This culminates in the synchronous release of each generation of spermatids into the tubular lumen in the process known as spermiation, which occurs at approximately 12.2-day intervals, coinciding with the entry of Al spermatogonia into the cellular differentiation process.3
Cross-sections of equine seminiferous tubules have revealed eight consistent cellular associations, or stages, of spermatogenesis, which account for essentially all of the developmental stages during spermatogenesis. Thus, in a given section of seminiferous tubule, each time all eight stages are completed in sequence, spermatozoa have been released into the seminiferous tubular lumen. This is known as the cycle of the seminiferous epithelium and has been described in detail for the stallion.3–5
Seasonal variations in numbers of spermatogonia, spermatocytes, and daily production of sperm have been described.4,5 Moreover, seasonal and age-related changes in Sertoli cells and Leydig cells may also contribute to variations in sperm production. Testicular parenchymal volume has been associated with sperm production such that, in most species studied, spermatogenic efficiency may be measured. For mature stallions, approximately 18–20 × 106 sperm per gram of testicular parenchyma has been estimated to be the daily production of sperm.6
SPERM TRANSPORT IN THE STALLION
As sperm are produced by the seminiferous tubules and released into the tubular lumen following spermiation, they are passively transported into the rete testis. This area is an extensively branched, centrally located storage reservoir for sperm and rete testis fluid. Both ends of each seminiferous tubule empty into the rete. The rete testis fuses with the 10–20 efferent tubules (vasa efferentia), which, in turn, fuse with the epididymal duct at the origin of the caput epididymis. The efferent tubules become progressively more convoluted as they reach the caput epididymis and merge into a single epididymal duct in which the sperm are received.7
The Epididymis
For purposes of description, the epididymis has been divided arbitrarily into three major anatomic regions with indiscreet borders: (1) caput (head), (2) corpus (body), and (3) cauda (tail); however, several additional functionally distinct regions, or zones, have been described.2,8 Additional sperm maturation occurs during epididymal transit, evidenced by the knowledge that caput epididymal sperm are incapable of fertilization and demonstrate little or no motility, whereas those residing in and leaving the cauda epididymis are fully fertile for most species studied. The latter also display a percentage of progressively motile sperm that is similar to that of ejaculated sperm.2,8
In the caput, the secretions of the epididymal epithelium essentially replace the rete testis fluid that accompanies the entering sperm and that is absorbed by the epididymal epithelium. Additional changes in epididymal fluid character occur as the sperm progress caudally through the epididymis. Changes in membrane phospholipids, proteins, and carbohydrates have been described in various regions of the epididymis.9 Sperm movement through the epididymis is thought to be independent of sperm motility factors and is more reliant on epididymal epithelial factors such as luminal ciliary activity and smooth muscular activity from the epididymal duct wall.10 This wall has a well-developed smooth muscle component that becomes progressively more prominent caudally along the length of the duct. The cauda epididymis is richly innervated by sympathetic neurons associated with smooth muscle cells.10 By contrast, the caput has sympathetic neurons but the nerve terminals are fewer and more likely to be associated with the microvasculature. It has been suggested that sperm passage from the caput to corpus epididymis takes approximately 4.1 days; however, transit time through the cauda is not known with certainty for the stallion because frequency of ejaculation modifies such transit.11 Total sperm transit time through the epididymis has been estimated at approximately 10 days for stallions. Frequent ejaculation may decrease transit time of sperm through the cauda by several days, resulting in decreased average storage time of individual sperm compared with that of sexually rested stallions.2
From the cauda epididymis, where the majority of sperm are stored, sperm enter the ductus deferens and remain there until smooth muscular contractions at ejaculation propel them to the pelvic and penile urethral regions. It has been estimated that the majority of extragonadal sperm are stored within the cauda epididymis and ductus deferens. Of the total sperm stored, approximately 61% is located within the cauda epididymis.12
THE EQUINE SPERM CELL
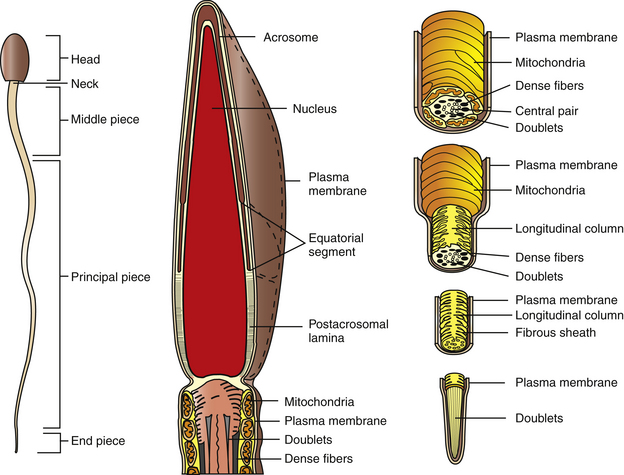
The equine sperm cell, like other mammalian sperm, is a highly specialized cell, consisting of a head, middle piece, principal piece, and end piece (Fig. 5-2). The posterior border of the sperm head and the middle piece are joined at the neck region, which is itself a highly specialized structure. Equine sperm heads are flattened, paddle-shaped cells with dimensions of approximately 60–65 μm in overall length; head length, 6–7 μm; middle piece length, 10 μm; principal piece length, 40 μm; end piece length, 4–5 μm. The sperm head width is approximately 3.5–4.0 μm at the equatorial segment of the acrosome, the widest dimension of the cell.9
The Plasma Membrane
The entire sperm cell is covered by the plasma membrane, which is a typical phospholipid bilayer incorporating cholesterol, complex carbohydrates, and proteins. Cholesterol likely serves as a membrane-stabilizing substance.13 Some of the proteins are embedded within the lipid layers to varying extents, but others are localized to the outer lipid leaflet and are associated with the glycocalyx. Certain transmembrane proteins have been reported to span the lipid bilayer as ion channels, pores, receptors, and signal transduction components. Proteins that function as receptors for various hormones and extracellular matrix components are localized on the outer lipid layer and glycocalyx, although transmembrane receptor proteins are likely to exist.13 Proteins make up about 50% of total membrane molecular weight.13 The plasma membrane appears to be anchored to underlying structures in the region of the sperm acrosome, post-acrosomal lamina, and neck region.
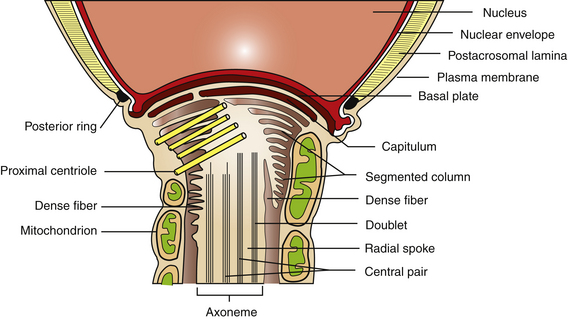
The plasma membrane overlying the sperm head has been subdivided into specialized surface domains, notably the acrosomal and postacrosomal regions.14 In the acrosomal region, the anterior portion has been described as the marginal segment and includes the peripheral rim of the cell and lies anterior to the principal segment, which is the major acrosomal area. The posterior part of the acrosome is called the equatorial segment and is the area in which sperm-zona pellucida binding is initiated during the early stages of fertilization. The marginal, principal, and equatorial segment domains comprise what is often called the acrosomal cap. The postacrosomal region of the plasma membrane is the region between the posterior margin of the equatorial segment of the acrosome and the neck of the sperm cell. At the junction of the neck and middle piece is the posterior ring, which probably forms a tight seal between cytoplasmic compartments of the head and tail of the sperm (Fig. 5-3).14
An interesting feature of the sperm cell is that of differential surface composition of membrane-associated constituents. It has been reported that the plasma membrane overlying the head differs in lipid and protein content from that of the sperm tail. In addition, the net negative charge of the sperm has been more closely associated with the tail than the head.14 Furthermore, the use of fluoresceinated lectin-binding sites and colloidal iron has demonstrated that surface-associated carbohydrates differ with respect to cellular location.15,16 In stallion sperm, similar findings have been reported with respect to surface carbohydrate variations.15
The Nucleus
The nucleus of the sperm cell consists of chromatin-containing genomic DNA, which is densely packed and closely associated with the major nuclear proteins known as protamines. The latter are low-molecular-weight proteins (27–65 amino acids) that are highly basic and rich in arginine and cysteine residues. Numerous disulfide bonds serve to stabilize the DNA-protamine complexes. Chromatin from normal sperm are somewhat resistant to acid-denaturation, which forms the basis of the sperm chromatin structure assay.17,18 Using this flow cytometric assay, it has been shown in numerous species, including horses, that males with subnormal fertility or infertility demonstrate characteristic fluorescence patterns of their sperm cell populations.17,18
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree