, Monika Hassel2 and Maura Grealy3
(1)
Centre of Organismal Studies, University of Heidelberg, Heidelberg, Germany
(2)
Spezielle Zoologie, Universität Marburg FB Biologie, Marburg, Germany
(3)
Pharmacology and Therapeutics, National University of Ireland Galway, Galway, Ireland
9.1 Start of Differentiation Programs in Accordance with Position
9.1.1 How Is Development in Accordance with Location (Normotopic Development) Accomplished in Spite of Genomic Equivalence?
In the course of embryonic development all cells are allocated the entire genetic information, as cell divisions are based on mitotic cell cycles. In the S phase of the cell cycle the DNA is faithfully replicated; the two daughter cells obtain the exact identical genetic information since mitochondria are also apportioned to both cells. Initially there is genomic equivalence, that is the cells of an embryo are initially genetically identical and totipotent (= capable of giving rise to any cell type; proof: nuclear transplantations, Chap. 13).
Yet, the tasks the descendants of a founder cell have to take over are not at all identical. Cells of the embryo must behave in accordance with their location. Here they construct the nervous system, there they form a muscle, at this place they must jointly produce an element of the skeleton giving it a distinct shape, and at that place they must commit suicide to create a cavity. How do cells know where they are? Do their genes somehow tell them what their position is at each moment? This is not possible, for the DNA of the nucleus does not contain a street map, and the cells of the embryo cannot look into their nuclei to find out where they are. Somehow position-dependent cell differentiation must be initiated from the outside. By “from outside” we mean by signals having their origin outside the nucleus of the cell in question.
Different tasks require switching on or off of different genes, as each cell type has its own set of proteins. The initially totipotent cells must make decisions about which genetic sub-programs to call into action.
9.1.2 Before Being Committed to a Distinct Fate, Cells Need Information About Their Location in the Embryo. Teratomas Show: Without Proper Positional Cues Chaos Will Arise
Teratomas are failed, chaotically organized embryos. They derive from unfertilized cells of the germ line in the testes or ovaries. Occasionally germ line cells start embryo development precociously. Another source of teratomas are fertilized eggs which fail to be taken up by the tube of the oviduct and instead implant anywhere in the abdominal cavity. Experimentally, in mice teratomas are produced by removing blastocysts from the uterus and implanting them into the abdominal cavity. (Teratomas may develop into malignant teratocarcinomas, tumours which even can metastasize. Cells derived from teratocarcinomas, such as the widely used murine 3T3 or F9 cells, are frequently immortal and can easily be propagated as cell cultures.) Teratoma cells are, as a rule, genetically still intact. If implanted into normal blastocysts, they may integrate into their new surroundings unobtrusively and participate in the construction of the new animal. In spite of intact genomic information chaos arises if a correct sequence of appropriate cues does not guide locality-pertinent utilization of genetic subprograms.
In its protein-coding genes the genome contains information about the order in which amino acids have to be linked to get a distinct protein. Beyond this basic information the genome is organized in such a way that entire programs can be called up to manufacture, for example, a muscle cell or a nerve cell (Sect. 12.2). But since the genomic information initially is identical in all cells, a cell needs positional information enabling it to behave in accordance with its location. This information cannot directly be derived from the cell’s own nucleus.
9.2 Defining the Body’s Coordinates
9.2.1 First the Body Axes (Antero-Posterior, Dorsal-Ventral) Must be Established; This Can Take Place in Oogenesis or Subsequent to Fertilization
The first decision to be made is the future location of the head and the tail, the back and belly. Most animals are bilaterally symmetrical (Fig. 9.1). Perpendicular to an antero-posterior axis extends a dorso-ventral axis, while the left and right sides are mirror-inverted symmetrical. Bilateral symmetry means capable of being split into two equal parts so that one part is a mirror image of the other. In developmental biology axes of asymmetry or anisotropy are called polarity axes.
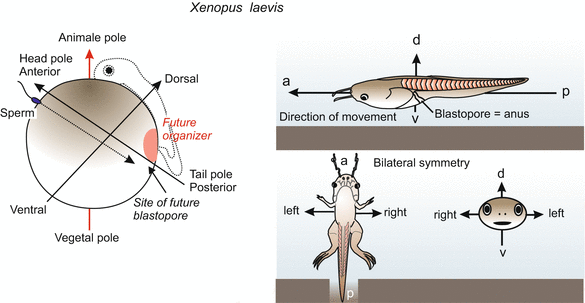

Fig. 9.1
Determination of bilateral symmetry in the amphibian embryo. Decisive for the specification of the antero-posterior and the dorso-ventral axis are (1) the site of sperm entry and migration path of the sperm centriole, and (2) gravity: When the contact with the sperm releases rotation of the egg cortex, gravity determines the direction of the rotation
Egg cells are always organized in a polar manner. Frequently, oocytes in the ovaries are not completely surrounded by nourishing nurse cells. The oocyte is fed predominantly from one side (Fig. 4.25 as an example), which suggests a source of cues for asymmetric organization. Even gravity has an influence in certain cases. Thus in the oocyte various substances, whether produced by the egg itself or provided by nurse cells, are not uniformly deposited. Special transport systems manage their asymmetric distribution as is known from the egg of Drosophila (Figs. 9.2 and 4.12). But also in the oocytes of other animals the internal constituents are not uniformly distributed. Heavy yolk granules often accumulate near the vegetal pole while the nucleus of the oocyte, in traditional terminology called the germinal vesicle, comes to lie near the animal pole, where the polar bodies are constricted off later in the course of the meiotic divisions (actually, the location of the polar bodies defines the animal pole).
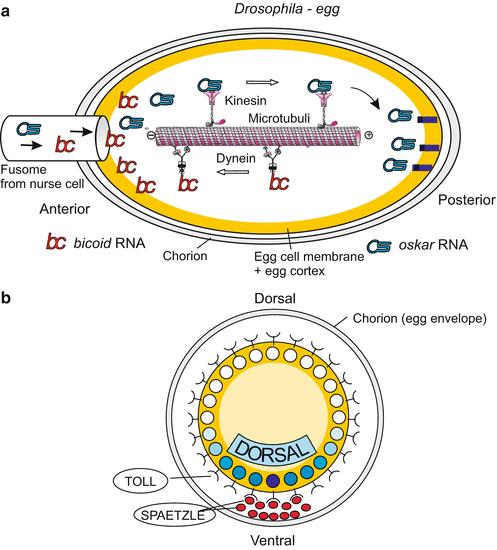

Fig. 9.2
(a, b) Maternal determinants of the body axes in Drosophila. (a) Antero-posterior axis: Of particular significance are transport systems which direct the diverse species of mRNA supplied by the nurse cells to different locations in the egg. The UTR (UnTranslated Region) of the transcripts serves as a postal code. Kinesin motor proteins transport oskar mRNA to the posterior egg pole where it is moored at the cytoskeleton. In turn, dynein motor proteins arrange accumulation of bicoid mRNA near the anterior pole. Many more components, not shown here, are involved in the specification and stabilization of the longitudinal body axis. (b) Dorso-ventral axis: localization of important maternal elements in the cross-sectioned egg
In most eggs it is not difficult to recognize the animal-vegetal axis under the microscope. But in the vast majority of egg cells only one unipolar axis is visible, not a complete bilaterally symmetrical architecture. The egg of Drosophila is exceptional, displaying a bilateral shape and internal organization (Fig. 9.2). Most animal eggs, however, display a recognizable animal-vegetal axis but neither a bilaterally symmetrical shape nor bilateral organization in their internal constituents.
The animal-vegetal axis may coincide with either the future antero-posterior or the dorso-ventral body axis, or with neither of them. In molluscs, ascidians and birds the animal-vegetal axis of the egg coincides with the dorso-ventral axis of the embryo. But this coincidence is merely rule of thumb and not a generally valid rule.
9.2.2 In Drosophila, the Mother Decides in Advance the Future Polarity Axes of Her Child; The Decisions Thus Become Dependent on Maternal Genes
In the invertebrate model Drosophila symmetry determination is markedly different than described below for vertebrates. A significant difference is that the process of specifying bilateral symmetry is completely under the control of the maternal genome. As Drosophila has a dominant position in developmental biology, there is the risk that peculiarities found in Drosophila are taken as a paradigm for animal development in general. In particular such fundamental decisions as the location in which the head, the tail, the back and the belly are to be formed, must be, so we are inclined to think, put under the control of the genome and Drosophila appears to testify the veracity of our intuitive assumption. Is it really so, that these fundamental decisions are laid down in our genome? If we have a closer look on the events in oogenesis even in Drosophila the decisive factors originate outside the egg cells.
The antero-posterior polarity axis is specified by the spatial distribution of particles enclosing maternally generated mRNA (RNP- particles). Bicoid message is deposited at the front pole, the mRNAs of nanos and oskar at the tail pole (Fig. 9.2). Viewed from the egg cell, the origin of these gene products is external, but the determinants are now internalized. When bicoid mRNA is translated into protein, this protein acquires the function of a transcription factor controlling gene activities in the early embryo (Chaps. 4 and 12). In addition, the embryo produces the TORSO receptors using maternally supplied torso mRNA; these receptors are exposed on the surface of the egg cell and recognize external cues. The cues are factors produced in the ovary by follicle cells and stored in the space between the egg cell and the egg envelope, in front of and behind the egg cell.
Likewise, specification of the dorso-ventral polarity is mediated by signal molecules that have been secreted by follicle cells at the future ventral side, stored in the ‘perivitelline’ space between egg cell and egg envelope, and picked up by receptors (coded by the maternal gene toll) of the egg cell membrane. The signals trigger a mechanism which redistributes a determinative cytoplasmic factor: The maternal factor DORSAL, initially homogenously distributed in the pre-cleavage egg cell, migrates into the ventral nuclei of the blastoderm (Fig. 9.2b; see also Fig. 4.35).
Since the messages for each of these factors (derived from bicoid, nanos, oskar and dorsal) and each of these receptors (derived from torso and toll) are products of genes, the Drosophila genome is directly involved in the establishment of the coordinates of the body. It is, however, the maternal genome that is involved, and not the embryo’s own (zygotic) genome. From the perspective of the oocyte, even in Drosophila the decisive cues for orientation come from outside, from the nurse cells and follicle cells of the maternal ovary.
It is genetics that testify the significance of maternal genes. Even if any one of the above mentioned genes is homozygously defective, the mother herself is not affected, because these genes are required only in oogenesis. The mother herself displays a normal phenotype, only the offspring manifests the mutation. One speaks of maternal-effect mutations or genes.
9.2.3 If the Coordinates Are Established Only After Fertilization, External Cues Can be Utilized: Amphibians as an Example
In the simplest case the sperm can play the role of an orientation guide, and in the round worm Caenorhabditis elegans the site where the sperm enters the egg marks the future posterior pole of the embryo (see Fig. 9.7). Also in vertebrates environmental cues are significant.
In amphibians (and in the zebrafish Danio) the animal-vegetal axis enables an observer to predict, approximately, where the head will be formed. The head will be located within a short radius of the animal ‘north’ pole (Figs. 9.1 and 9.3). The spine will extend over the animal hemisphere, cross the equator and end at about the Tropic of Capricorn. But initially it is not determined along which of the 360 possible longitudinal lines the spine will extend. The position of this ‘zero meridian’ is determined at the moment of fertilization. Two external parameters are involved: the point of sperm entry and gravity.
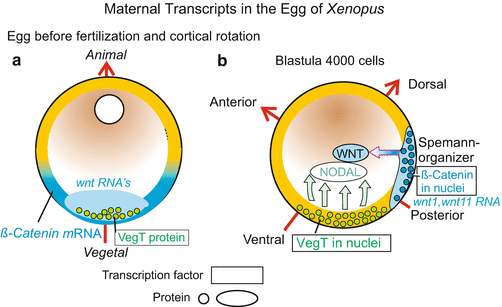

Fig. 9.3




(a, b) Significance of maternal transcripts for establishing the body coordinates (polarity axes) in the embryo of Xenopus. (a) Distribution before cortical rotation. (b) After redistribution of the maternal transcripts (mRNA) subsequent to fertilization, the future bilateral symmetry of the amphibian embryo is specified. In addition, the region in which β-catenin is taken up into the nuclei determines the future position of the blastopore and of the overlying Spemann organizer
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


