Chapter 4 Specific Syndromes Causing Acute Intrinsic Renal Failure
Ethylene Glycol (EG) Toxicity
Introduction and Pathophysiology
A. Consumption of EG is an important cause of acute intrinsic renal failure (AIRF) in dogs and cats, and should always be considered in geographic regions and during times of the year in which antifreeze is used. Ingestion usually occurs as a consequence of improper storage or disposal of radiator fluid. It is the most common cause of AIRF in most veterinary practices, depending on the region.
B. EG toxicity is dose dependent.
1. The minimal lethal dose in dogs is 4.4 to 13.2 mL/kg. Dogs that rapidly ingest large doses of EG frequently vomit, which may limit exposure.
C. Some brands of antifreeze contain propylene glycol as a replacement for EG. Propylene glycol does not cause nephrotoxicity, but its ingestion can be confused with EG intoxication because both increase the osmolal gap, result in positive reactions on the EG Test Kit (PRN Laboratories, Pensacola, Fla.) as well as cause central nervous system (CNS) depression, osmotic diuresis, and metabolic acidosis with increased anion gap.
E. EG is readily absorbed from the gastrointestinal (GI) tract within 1 hour after ingestion and achieves peak serum concentrations in dogs and cats 3 hours later. EG remains detectable in the circulation for at least 12 hours, but usually cannot be detected 48 hours after ingestion.
1. EG rapidly disappears from the circulation due to a combination of renal clearance and metabolism.
3. About 50% of EG is excreted unchanged into the urine in animals with normal renal function. Peak concentrations of EG in urine occur approximately 6 hours after ingestion in dogs. Delayed renal clearance may occur in animals with pre-existing renal dysfunction, which may magnify EG toxicity because more conversion to cytotoxic metabolites may occur.
F. Dogs and cats of any age can be poisoned by EG but animals younger than 6 months of age may be less susceptible to permanent renal injury.
G. Unmetabolized EG is not very toxic. CNS depression can arise from hyperosmolality as EG is absorbed from the GI tract. Vomiting, which frequently occurs soon after EG ingestion, may be caused by direct mucosal irritation or may be a due to the rapid increase in plasma osmolality. The polyuria that occurs soon after ingestion of EG is a result of osmotic diuresis.
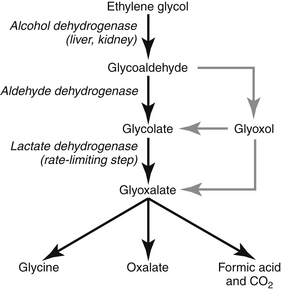
H. Hepatic metabolism of EG rapidly produces circulating metabolites that are extremely toxic (see Figure 4-1). Renal metabolism of EG may result in local accumulation of nephrotoxic metabolites. The toxic metabolites of EG (in order of decreasing toxicity) are:
I. Large quantities of acid are produced as EG is metabolized. The resultant metabolic acidosis can be life-threatening.
J. Oxalate crystal accumulation in tissues is a marker of EG poisoning and not a major cause of tissue damage or organ failure.
K. All of the hallmark findings of EG poisoning can be reproduced by the metabolites of EG without oxalate accumulation.
L. The half-life of EG is less than 12 hours in dogs and less than 2 to 5 hours in cats; soon after ingestion it metabolizes to its very harmful end products.
M. Cats develop EG toxicity at lower dosages and develop crystalluria and renal failure earlier than do dogs. The metabolism of EG may be more rapid in cats than in dogs, and feline renal tubular cells may be more sensitive to the cytotoxic effects of EG metabolites.
N. The enzyme alcohol dehydrogenase (primarily in the liver) is important in the initial degradation of EG. This enzyme can be inhibited pharmacologically in an attempt to reduce production of toxic metabolites. Alcohol dehydrogenase also is located in the kidney, which may account for local generation of cytotoxic metabolites.
O. Clinically, the syndrome occurs in three phases each affecting a different body system: CNS, cardiopulmonary, and renal. Death can occur during any of these phases.
1. Central nervous system signs can be seen within 30 minutes of ingestion and persist for 12 hours.
a. Clinical signs are attributed to cytotoxic metabolites. Hyperkalemia, hypocalcemia, and metabolic acidosis also may contribute.
2. Cardiopulmonary signs can develop at a variable time after EG ingestion depending on how much was ingested.
a. Cardiopulmonary involvement may not be seen as a separate phase and may be obscured by initially severe CNS involvement and developing renal failure.
3. Renal involvement can be detected within hours of EG ingestion, but confirmation of renal failure based on increased serum creatinine and blood urea nitrogen (BUN) concentrations is not apparent until 24 hours after ingestion.
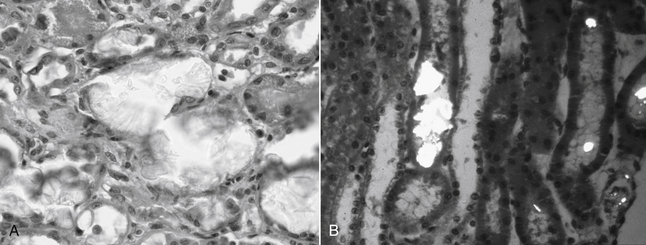
b. Obvious light microscopic lesions in the kidney are not identified until after 72 hours in some patients (evidence for ultrastructural damage occurs within hours) (Figure 4-2):
(1) Dilated proximal tubules, due to downstream obstruction of the nephron by calcium oxalate crystals.
Diagnosis of Ethylene Glycol Poisoning
History
1. Direct observation of consumption of the toxin may be made before the onset of any clinical signs, but this is not common. When exposure to EG is described by the owner, try to estimate the volume consumed and the time interval from consumption until presentation to your office, because the outcome for EG intoxication is both dose- and time-dependent. Prognosis is directly affected by this knowledge.
2. Owners should specifically be asked about access to EG when an acutely and severely ill dog or cat is presented in late fall or spring in geographical regions in which radiator fluid is used. Questions such as, “Did you or your neighbors recently change radiator fluid?” and “Are open containers of EG kept anywhere on the premises, such as in the garage?” are appropriate.
3. History will vary according to the stage of poisoning observed by the owner and the amount of EG ingested.
a. Early effects after ingestion (within 6-8 hours).
(1) CNS effects.
(a) Inebriation may be the earliest finding. The animal may suddenly appear confused or depressed, with staggering and incoordination due to the direct effects of unmetabolized EG.
(b) Severe depression or rapid progression to coma (“acute collapse”) may occur due to the metabolic effects of EG and the metabolic acidosis associated with its metabolism.
(c) Seizures may be due to rapid onset of hypocalcemia as the oxalates generated by metabolism of EG chelate calcium.
(3) Urinary effects.
(a) Polyuria may be noted soon after ingestion of EG due to the osmotic effects of rapid GI absorption of EG and its subsequent filtration through the kidneys, which results in osmotic diuresis. EG (similar to ethanol) also may inhibit antidiuretic hormone, which may contribute to diuresis.
(c) Polydipsia can result from a sudden increase in serum osmolality after rapid absorption of EG and stimulation of osmoreceptors in the hypothalamus, an effect that is pronounced in dogs during the first hour after ingestion. Polydipsia can persist beyond this stage because dehydration and volume depletion stimulate water consumption until the animal becomes severely depressed.
b. Later effects after ingestion (12-24 hours or longer) are largely attributable to renal failure and include vomiting, diarrhea, and depression. The animal may appear in pain when picked up or may be reluctant to move because of pain arising from swollen muscles or kidneys.
(2) Urine volume at this later stage usually is severely decreased, often nearly to the point of anuria.
Physical Examination
C. Dehydration is common due to polyuria accompanied by anorexia and vomiting, especially if the animal is presented 24 hours or more after ingestion of EG.
D. Signs of cardiopulmonary failure may be present in severely affected animals.
1. Tachypnea, increased bronchovesicular sounds or crackles associated with pulmonary edema or sterile bronchopneumonia.
E. Palpation of the costovertebral angles may disclose painful kidneys or muscle pain. The kidneys may be swollen or normal in size.
F. Uremic oral ulcers and foul oral odor may be present. A sweet oral odor may be detected immediately after consumption of EG.
Laboratory Findings in Ethylene Glycol Poisoning
A. Abnormalities will be present on urinalysis soon after ingestion of EG (as early as 4 to 6 hours after ingestion).
3. Calcium oxalate crystals may be seen on urinary sediment examination.
a. Often noted in experimental studies in animals but reported in less than 50% of naturally occurring cases.
c. Oxalate crystals may be observed in the urine of normal dogs and cats as a result of diet, and their presence therefore is not pathognomonic for EG poisoning. Their presence however is supportive of the diagnosis in the appropriate clinical setting.
4. Unidentified crystals often are reported from the laboratory in animals with EG poisoning.
a. These unidentified crystals initially were thought to be hippurate, an alternative pathway metabolite.
B. Measured urine output.
1. Very small urine volume is a hallmark of EG poisoning 12 to 24 hours after ingestion of a toxic dose.
C. Serum biochemistry.
1. BUN and serum creatinine concentrations usually are increased by the time clinical signs have developed and the animal is presented to the veterinarian for evaluation.
a. Some of the azotemia may be prerenal as a consequence of dehydration, but most of it is primary renal azotemia by 12 hours after EG ingestion.
b. BUN and serum creatinine concentrations are normal in animals that are presented very soon after EG ingestion (within 8 to 12 hours).
2. Serum phosphorus concentrations may be disproportionately high compared with BUN and serum creatinine concentrations, possibly due to absorption from the GI tract of phosphate-containing rust inhibitors that are added to some antifreeze formulations. Later, hyperphosphatemia is primarily a consequence of renal failure.
3. Serum osmolality may be dramatically increased soon after EG ingestion due to the presence of unmetabolized EG.
a. Measured serum osmolality must be determined by an osmometer and should not be confused with the calculated osmolality that is supplied with serum biochemistry results by many commercial laboratories.
b. Calculated osmolality (mOsm/kg) may be determined by the following formula: 2(Na + K) + glucose/18 + BUN/2.8.
c. The difference between measured and calculated serum osmolality has been referred to as the osmolal gap. Normally, calculated serum osmolality is similar to measured serum osmolality and the normal osmolal gap is < 10 mOsm/kg.
d. EG is rapidly absorbed from the GI tract into the blood and increases measured serum osmolality. Calculated serum osmolality, however, is unchanged because EG is not a component of the equation for calculated osmolality. Thus, EG ingestion increases the osmolal gap.
e. A high osmolal gap (often > 50 mOsm/kg) is common in early EG poisoning in dogs and cats. Values of > 100 mOsm/kg are not observed in any other disease in veterinary medicine.
f. In animals treated with ethyl alcohol, this solute also will contribute to serum osmolality measured by freezing point depression osmometry and will contribute to the increased osmolal gap. Ethyl alcohol is a volatile solute and will not contribute to osmolality measured by vapor point elevation osmometry.
4. Serum potassium concentration may be increased, and hyperkalemia, if severe, can be life-threatening.
a. Early in the course of the intoxication, hyperkalemia may occur as a consequence of severe metabolic acidosis associated with EG metabolism.
5. Serum calcium concentration often is decreased as a result of several factors:
d. As renal failure becomes established, hyperphosphatemia contributes to a reciprocal fall in serum calcium concentration.
6. Detection of EG.
a Measurements of serum and urine EG concentration are not available from most commercial diagnostic laboratories.
b. High concentrations of EG in either serum or urine will confirm poisoning. EG however does not persist long, and concentrations may fall below detectable levels before clinical samples are obtained for analysis.
c. The EGTest Kit is an in-house test kit designed for veterinarians to allow rapid detection of unmetabolized EG.
(1) A colorimetric reaction based on generation of formaldehyde in the presence of EG will identify concentrations as low as 50 mg/dL.
(2) False-negative reactions can occur with patient samples obtained less than 30 minutes or more than 12 hours after ingestion.
8. Blood gas analysis may support a diagnosis of EG poisoning.
a. The metabolites of EG result in severe metabolic acidosis early in the course of intoxication (within hours).
b. Later, as renal failure becomes established, decreased excretion of acid by the kidneys contributes to the acidosis.
c. Respiratory compensation (hyperventilation) and decreased pCO2 are expected unless cardiopulmonary complications are present or the animal is comatose.
d. A high anion gap is supportive of EG intoxication.
(1) The increased anion gap occurs due to titration of bicarbonate by acid metabolites of EG and accumulation of the associated unmeasured anions (e.g., glycoaldehyde, glyoxylate).
(2) The largest increase in anion gap occurs early, before renal failure develops. Phosphates and sulfates contribute to the anion gap after renal failure has been established.
D. Plain radiographs show normal to slightly enlarged kidneys. Excretory urography is contraindicated.
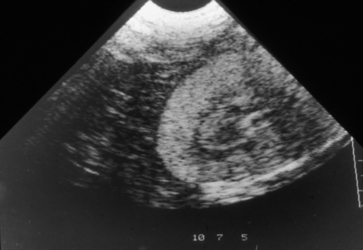
E. Ultrasonography may disclose abnormalities soon after EG ingestion (Figure 4-3)
1. Renal cortical echogenicity is increased by 3 to 4 hours after ingestion in experimental studies in dogs.
2. Renal medullary echogenicity is increased by 5 hours after ingestion and continues to increase until 8 to 10 hours after ingestion.
F. Renal biopsy findings vary according to the time interval after ingestion of EG.
4. When biopsied less than 72 hours after ingestion, renal lesions may be minimal. Interstitial edema may be noted in some cases.
5. When biopsied more than 7-10 days after ingestion signs of healing by regeneration or healing by fibrosis and mononuclear cell infiltration may be observed.
Treatment
A. The time interval for starting treatment is extremely critical because the half-life of EG is very short.
B. Therapy differs according the time that has elapsed between ingestion of EG and presentation to the veterinarian.
1. Induction of vomiting and gastric lavage should be considered in animals presented within 1 to 2 hours after EG ingestion. These procedures are not considered if the animal is severely depressed or comatose because of increased risk for aspiration. Administration of activated charcoal is of questionable value because EG is not well adsorbed by charcoal. The effect of activated charcoal on adsorption of intermediary metabolites in the intestinal lumen after enterohepatic recirculation is not well known.
2. Animals presented within 24 hours of probable ingestion should have therapy directed against any further metabolism of EG, measures taken to enhance EG excretion or removal from the body, and supportive measures to help combat metabolic abnormalities already present.
3. Animals presented more than 24 hours after ingestion are not likely to benefit from therapy aimed at reducing metabolism of EG because most of the parent compound will have already been metabolized. If however EG is still detectable in body fluids, antidote treatment should be employed. Alternatively, supportive measures to combat metabolic disturbances should be carried out.
C. Multiple approaches to treatment should be employed to increase the likelihood of survival, especially in animals in the early phases of EG poisoning.
D. Place an indwelling IV catheter. Even if the animal is presented early and does not appear critically ill, an IV line still should be placed because these patients can deteriorate rapidly and an IV catheter is the only reliable way to provide proper management for patients with complicated metabolic disturbances.
G. Correct hypocalcemia, as necessary to control tetany or seizures, with infusions of calcium gluconate or calcium chloride.
H. Correct metabolic acidosis immediately.
2. Infuse sodium bicarbonate as needed to correct acidemia, ideally by monitoring serial blood gas determinations.
3. Ongoing acid production from continued metabolism of EG and acid retention as a consequence of developing renal failure can make the management of metabolic acidosis challenging.
I. Decrease breakdown of EG to toxic metabolites.
1. Increased survival of dogs and cats poisoned with EG in experimental studies was demonstrated when early treatment with ethanol was provided. Similar results were demonstrated with 4-methylpyrazole (4-MP; fomepizole) in experimental studies of dogs poisoned with EG.
2. Both ethanol and 4-MP compete with EG as a substrate for the enzyme alcohol dehydrogenase, thus decreasing its metabolism to toxic compounds. This treatment results in higher plasma concentrations of EG and increased excretion of unchanged EG into urine when renal function is normal. Treatment with ethanol or 4-MP within 24 hours of ingestion is warranted.
3. Ethanol therapy.
a. The goal of treatment is to maintain ethanol concentrations high enough to effectively compete with EG as a substrate for alcohol dehydrogenase. The required ethanol concentration will vary with the individual patient and amount of EG ingested. Ethanol concentrations of 100 mg/dL are recommended for efficacy in human medicine but commercial veterinary diagnostic laboratories do not routinely provide this measurement. Ethanol concentrations > 60 mg/dL are necessary for effective treatment of cats whereas concentrations as low as 35 mg/dL may provide effective inhibition of EG metabolism in dogs.
c. The traditional high dose protocol for dogs consists of 5.5 mL/kg of a 20% ethanol solution IV every 4 hours for 5 treatments and then every 6 hours for 4 treatments. This protocol is equivalent to 1.1 g/kg of ethanol given intermittently.
d. Constant rate infusion (CRI) of ethanol after administration of a loading dose is likely to be superior pharmacologically. This method has not been reported in the veterinary literature but it has been used successfully in our hospital:
e. The following low dose CRI protocol for dogs is designed to maintain ethanol concentrations of 50 mg/dL using 30% ethanol:
f. The dosage of ethanol for cats is 5 mL/kg intraperitoneally using a 20% ethanol solution and given every 6 hours for 4 treatments and then every 8 hours for 4 treatments. Although this regimen was developed in experimental studies of cats, it has been modified for IV use in cats:
g. Ethanol can be given orally by the owner in an emergency before departing for a veterinary hospital if the evidence for EG ingestion is conclusive. The dose is 1.0 to 1.4 mL/kg of a 40% (i.e., 80 proof) alcoholic beverage (e.g., vodka). Vomiting may occur after rapid administration of this dose.
4. 4-Methylpyrazole therapy.
a. Marketed commercially as Antizol-Vet, 4-MP is a specific antidote for EG poisoning if given early enough after ingestion.
b. Rescue with 4-MP is superior to that achieved with ethanol in dogs and it does not produce CNS depression.
d. 4-MP is less effective in cats than in dogs when at standard dosages. Neither 4-MP nor ethanol rescue is very effective 3 hours after a lethal dose of EG in cats, but ethanol appears to be superior to 4-MP.
f. Provide supplemental thiamine and pyridoxine to encourage metabolism to less harmful intermediates by alternate metabolic pathways. The value of this theoretical treatment has not been proven.
J. Induce diuresis with IV fluids and some combination of furosemide, mannitol, and dopamine.
1. Increasing or maintaining glomerular filtration rate (GFR) will facilitate renal excretion of EG and its metabolites.
2. Mannitol may be the diuretic of choice before anuria has become established because of its superior effects on renal edema. Mannitol will be detected when serum osmolality is measured using either freezing point depression or vapor point elevation osmometry.
K. Eliminate EG from the body.
2. Induce emesis if ingestion of EG has occurred recently and the patient does not have CNS depression or coma (i.e., high risk of aspiration).
3. Administer an aqueous slurry of activated charcoal (5 g/kg) via stomach tube if the animal is not severely depressed. Otherwise the risk for aspiration increases.
a. Some EG still in the lumen of the GI tract may be adsorbed and no longer available for absorption.
b. EG may undergo some enterohepatic circulation and be bound by charcoal even after it was initially absorbed into the body.
c. Improved survival has been reported in dogs with experimental EG poisoning when activated charcoal was added to standard therapy consisting of bicarbonate, ethanol, and fluids.
d. Commercially available activated charcoal may contain propylene glycol and glycerol. Absorption of these vehicles can increase measured serum osmolality and osmolal gap, and cause confusion in the diagnosis of EG intoxication. A positive test result on the EG test kit can result from the propylene glycol.
4. Dialysis is beneficial in removing EG and its toxic metabolites.
a. Consider temporary peritoneal dialysis for several exchanges of peritoneal fluid. Dialysis may be necessary for less than 24 hours if started early enough.
c. Long-term dialysis can result in recovery from EG nephrotoxicity but dialysis may be necessary for an inordinately long period of time (6 to 9 months).
d. Short-term hemodialysis removes both EG and toxic metabolites and may allow survival by decreasing nephrotoxicity after EG metabolism.
e. Long-term dialysis may be helpful or necessary to adequately manage animals with EG intoxication. Overhydration, hyperkalemia, severe metabolic acidosis, and retention of uremic toxins not manageable by conventional medical therapy will require dialysis if the patient is to have a chance for survival.
Prognosis for Prevention of Acute Intrinsic Renal Failure from Ethylene Glycol Poisoning
A. Ultimately depends on the amount of EG consumed and the time from ingestion until definitive therapy is started.
B. Survival was 12% in one large study of dogs and cats with EG poisoning. Half of the survivors were nonazotemic animals treated less than 12 hours after EG ingestion whereas the other half were in renal failure but all were younger than 6 months of age. Thus, young age may be a factor favoring survival.
E. Prognosis for recovery from intrinsic renal failure.
1. Prognosis should be considered grave if persistent oliguria or anuria characterizes the initial course after IV fluids.
2. Prognosis is grave for animals presented with established AIRF and more than 24 hours after ingestion of EG.
3. Prognosis is grave for any animal that develops progressive azotemia and severe oliguria despite adequate medical therapy, especially if complicated by hyperkalemia, severe metabolic acidosis, or overhydration.
Leptospirosis
Introduction
A. Leptospirosis is a common infection in dogs based on serologic evidence of exposure of dogs. Infection in cats does not result in recognized clinical disease, but serologic evidence of exposure exists in cats.
2. Leptospiral organisms are thin, coil-shaped, gram-negative, aerobic, and microaerophilic. Pathogenic strains are classified as Leptospira interrogans or L. kirschneri with at least 8 serovars in dogs and cats.
3. Classically, clinically relevant infections have been attributed to serovars L. canicola and L. icterohemorrhagiae.
4. Recently, clinical cases have most often been associated with infection by serovars L. pomona or L. grippotyphosa. Serovars Lbratislava, Laustralis, and others may be the causative agent depending on locale.
5. Diagnosis of leptospirosis has been increasingly made at veterinary teaching hospitals and diagnostic laboratories throughout the world over the past two decades.
6. Infection with specific serovars varies by geographic location:
b. In New Jersey and Michigan, L. pomona, L. grippotyphosa, and L. autumnalis are most common and L. bratislava is not reported.
f. In Ontario, Canada, L. autumnalis is most common, but L. bratislava, L. grippotyphosa, and L. pomona also occur.
B. Hosts that have adapted to leptospiral serovars act as a reservoir for maintenance of the infection. Clinical signs either are mild or inapparent in adapted hosts. Clinical signs are more severe in animals that are not adapted to the infecting serovar (Table 4-1).
C. Source of leptospiral transmission and pathogenesis.
D. Clinical manifestations of leptospirosis depend on several factors.
4. Serologic response to previous vaccination (if any). Vaccination against serovars L. canicola and Licterohaemorrhagiae decreases the likelihood of clinical disease with these serovars.
E. Syndromes.
1. The peracute syndrome is a fulminant form of the disease, most often seen in puppies, that causes septicemia and death.
TABLE 4-1 Leptospiral Organisms and Their Associated Domestic or Wildlife Host
| Serovar | Domestic (Wildlife) Host |
|---|---|
| L. icterohaemorrhagiae | Dog (rat) |
| L. canicola | Dog |
| L. pomona | Cattle, pig (deer, skunk, opossum) |
| L. hardjo | Cattle |
| L. grippotyphosa | Cattle, (raccoon, opossum) |
| L. autumnalis | Mice |
| L. bratislava | Horse, swine (rat, raccoon, opossum, skunk, vole) |
| L. bataviae | Dog (rat, mouse) |
2. The acute systemic syndrome causes septicemia and severe illness with localization of organisms to various organs resulting in:
3. The subacute syndrome is a less severe form of the disease in which renal failure may be the only obvious clinical manifestation.
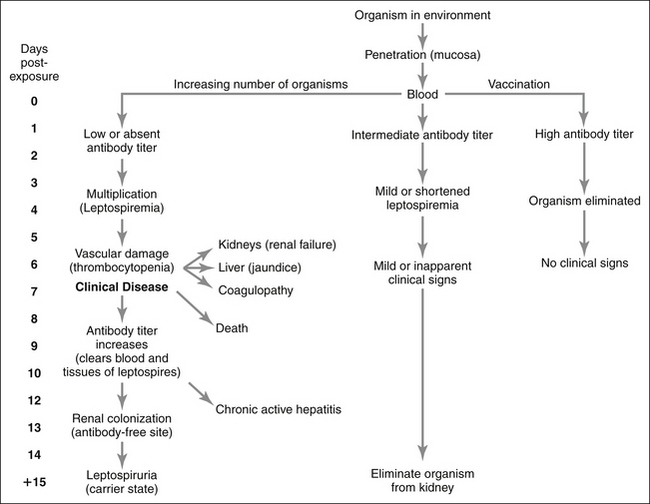
Pathophysiology (Figure 4-4)
A. Clinical signs are either inapparent or mild in animals that already have high or intermediate titers from previous vaccination against the infecting strain.
B. Organisms initially penetrate mucous membranes, often after the animal drinks contaminated water.
F. Damage to vascular endothelium, liver, and kidney occur. The severity of this damage depends on the virulence of the infecting serovar.
2. Organisms penetrate blood vessels to enter the renal interstitium and then renal tubular cells and tubular fluid. Mononuclear interstitial inflammation occurs in response to the invading organisms. Interstitial hemorrhage and edema as well as multifocal tubular necrosis may occur as a consequence of ischemia and toxins from the organisms. Defects in excretory renal function and urinary concentrating ability develop at this time.
G. During the second week after exposure, antibodies against the infecting serovar are produced and the organisms are cleared from the bloodstream and most tissues except for the kidneys and eyes.
I. Due to the absence of antibodies in the kidneys, organisms are not rapidly cleared from the kidneys and a carrier state develops in which the organisms live in the kidneys after recovery from initial nephritis.
J. The carrier state may last for months to years and is much more common than previously appreciated based on recent polymerase chain reaction (PCR) data. The carrier state may arise after clinically obvious illness or after recovery from an inapparent infection.
Signalment
History
Laboratory Evaluation
B. Coagulation studies.
3. Disseminated intravascular coagulation (DIC) is suspected in some patients by the presence of fibrin degradation products. Some serovars (e.g., L. australis) are more likely than others to be associated with DIC.
C. Serum chemistry.
1. Increased BUN and serum creatinine concentrations.
a. Early increases are due to prerenal azotemia and later increases to both prerenal and primary (intrinsic) renal azotemia.
3. Serum alkaline phosphatase (ALP) activity usually is increased, even in milder cases without icterus.
D. Urinalysis findings depend on the extent of renal involvement.




1. Urine specific gravity (USG).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree