Chapter 11 Soft Tissue Surgery
General Surgical Principles in Ferrets
As with dogs and cats, appropriate preoperative patient preparation includes placing an intravenous or intraosseous catheter. Provide intraoperative crystalloid fluid therapy at the rate standard for small animals (5-10 mL/kg per hour) as well as appropriate antibiotic coverage when indicated. Appropriate pain management is paramount and can be provided with opiods, nonsteroidal anti-inflammatory drugs, epidurals, and local blocks or a combination thereof (see Chapter 31).
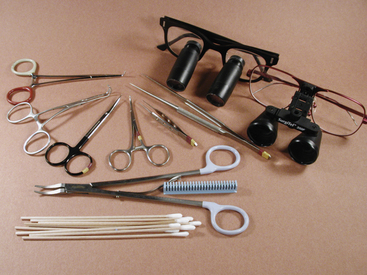
In performing surgical procedures in ferrets, the use of some specialized equipment (which should be kept on hand) is highly recommended. Endotracheal tubes in sizes from 2.5 to 4.0 mm should be available. Microsurgical instrumentation and magnifying loupes greatly simplify surgical procedures (Fig. 11-1). The surgical suite should be stocked with a variety of synthetic absorbable suture materials ranging in size from 4-0 to 6-0 with both cutting and taper needles. Various forms of hemostasis, including monopolar and bipolar radiosurgical units, absorbable hemostatic material such as Gelfoam (Pfizer Inc., New York, NY) and Surgicel (Ethicon, Inc., Johnson and Johnson Healthcare, Piscataway, NJ), or vascular sealing devices should be available. Equip the surgical preparation area, surgical suite, and recovery area with devices needed to maintain the ferret’s body temperature throughout the anesthetic procedure. Circulating hot-water blankets, warm-air circulators, fluid warmers for intravenous fluids, and warm water bottles may be used. Heat lamps can burn patients and should be used with caution. Self-adherent clear plastic drapes are useful to allow visibility of these small patients during surgery. These drapes also aid in keeping patients warm and dry and can eliminate the need for towel clamps. Monitoring should include pulse oximetry, electrocardiography (ECG), blood pressure, and body temperature.
Surgery of Cutaneous Neoplasia
Ferrets commonly present with a variety of cutaneous neoplasms (see Chapter 9); cutaneous neoplasia has been reported to be the third most common tumor type in ferrets.21 While some references have indicated that the most common dermal neoplasms are, in order, basal cell tumors, mast cell tumors, and squamous cell carcinomas, other retrospective studies have found fibromas to be the third most common tumor type, after basal cell tumors and mast cell tumors.31 Other less commonly identified cutaneous and subcutaneous tumors types include cutaneous hemangioma, hemangiosarcoma, lymphosarcoma, adenocarcinoma, piloleiomyosarcoma, rhabdomyosarcoma, neurofibroma, and cutaneous melanoma.18,31,36,47 Two cases of subcutaneous neoplasms histologically consistent with adrenocortical neoplasia have been reported; because these tumors were located along the ventral midline in young, spayed female ferrets, the authors speculated that ovarian or adrenal tissue had been seeded during ovariohysterectomy and later underwent malignant transformation.42
Signalment, history, and physical examination findings depend on tumor type. In one retrospective study, the mean age of presentation was 4.3 years31 with a range of 8 months to 9 years and slightly higher prevalence in females than in males (58% vs. 42%).
Despite the generally benign nature of most ferret cutaneous neoplasms, do not omit appropriate preoperative workup for potential neoplastic disease. Hematologic testing, plasma biochemical analysis, abdominal ultrasound, and thoracic radiographs to screen for concurrent diseases and anesthetic suitability should be considered, depending on the case and presentation. When appropriate, further diagnostic tests may include bone marrow aspiration as well as regional lymph node aspiration or biopsy, computed tomography, or magnetic resonance imaging. Obtain fine-needle aspirates of the mass or masses and submit samples for cytologic evaluation to plan appropriate surgical margins and provide owners with prognostic information before surgery. While cytologic examination may not provide a definitive diagnosis, a general cell type as well as distinction between malignant and benign processes can often be appreciated.2,25 Fine-needle aspirates can generally be performed with sedation, restraint, and local blocks; to prevent tumor seeding, plan approaches such that the needle tract is through areas slated for removal. Full histologic analysis is recommended for all excised tumors to confirm the diagnosis.
Surgery of cutaneous and subcutaneous neoplasia in ferrets is similar to that in other species. A variety of surgical modalities can be used to successfully remove cutaneous neoplasms. Scalpel, laser, and radiosurgical techniques have been described; a full review is beyond the scope of this chapter and the reader is directed to one of the excellent review articles for further information.25 Regardless of the technique used, pay attention to gentle tissue handling and rapid hemostasis. Take care during patient preparation not to spread or degranulate volatile masses such as mast cell tumors; therefore avoid aggressive palpation or scrubbing.43 Surgical margins depend on the tumor type and location. Cryosurgery has also been used for superficial tumors. Cryosurgery utilizes freezing/thawing cycles to destroy cancerous cells through the generation of ice crystals within the cells. Generally, two freeze/thaw cycles are recommended.17 Because of the extensive tissue necrosis adjacent to the mass, closure is usually not performed; for the same reason, make efforts to obtain a definitive diagnosis before cryosurgical treatment. Postoperative care, including pain control when required, is similar to that implemented in other species. As ferrets are not amenable to Elizabethan collars, we generally perform a subcuticular closure with a small-gauge (4-0) absorbable suture in resecting dermal masses. Overnight hospitalization is rarely required unless the mass was very large.
Exploratory Laparotomy
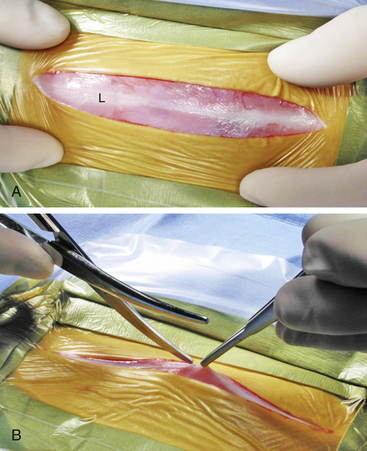
For a complete exploratory laparotomy, make a ventral midline incision beginning at the xiphoid and extending to the pubis (in the female) or to just cranial to the prepuce and extending parapreputial if needed (in the male). Ferrets have relatively thin skin and little subcutaneous tissue, so a light touch with the scalpel blade is warranted. The linea alba of ferrets is a wide, thin structure that is easily identified (Fig. 11-2, A). Gently grasp the linea with forceps and lift it away from the abdominal contents before penetrating the abdomen with a No. 15 scalpel blade. Extend the initial incision in the linea with scissors or a scalpel blade (Fig. 11-2, B). Be extremely cautious in penetrating the abdominal cavity or extending the incision to avoid damaging dilated or enlarged organs (most commonly the spleen). Also, so as not to penetrate the diaphragm, use caution in extending the incision in the linea cranially. To help gain exposure to the abdominal cavity, use small Balfour retractors with moistened gauze sponges. As with any exploratory laparotomy, use a thorough and systematic approach to examine the entire abdominal cavity. Remember that hypothermia is a major intraoperative complication, especially when a large abdominal incision is required. Monitor body temperature and be prepared to utilize multiple methods of thermal support, including forced warm-air mechanisms, overhead light/heat support, and very warm abdominal saline lavage. After completing the procedure, close the linea with 3-0 or 4-0 absorbable monofilament suture material (for example, polydioxanone [PDS]) in a simple continuous or interrupted pattern. Close subcutaneous tissues with fine absorbable suture material. A subcuticular pattern may be used to complete the closure. Alternatively, cyanoacrylic tissue glue may be used by applying the material after opposing the skin edges. Do not place tissue glue within the subcutaneous tissues because it elicits a foreign-body reaction. Skin sutures may also be used.
Gastrointestinal System
Salivary Mucocele Resection
Ferrets have five pairs of salivary glands: parotid, mandibular, sublingual, molar (buccal), and zygomatic.16 Although mucoceles are uncommon, they have been reported in ferrets (see Chapter 3).3,26,28 The zygomatic and molar (buccal) glands are most commonly affected. Affected ferrets are presented with unilateral facial or periorbital swelling or exophthalmos, and a fine-needle aspirate of the mass reveals a thick, mucoid fluid. Aspiration may provide temporary relief from the swelling, but treatment should involve marsupialization or surgical removal of the involved gland or glands. A thorough knowledge of the regional anatomy is required so that all of the involved glandular material is removed. As described in dogs, the zygomatic arch can be partially resected to facilitate exposure of the zygomatic gland. Although there are few reports of mucoceles in ferrets, the prognosis appears to be good after surgical removal of the affected gland.3,26,28
Mucoliths are common in the parotid salivary gland of ferrets. Although these are generally asymptomatic, affected ferrets are used as models in studies of human salivary mucoliths.45,46
Intestinal Surgery
One of the most common surgeries performed in young ferrets is removal of ingested foreign material from the stomach and small intestine. Foam rubber sponges, rubber objects, and cork are the most common foreign bodies removed.27 Most ingested foreign material becomes lodged in the small intestine or stomach, but esophageal foreign bodies have been reported.9,27 Trichobezoars (hairball foreign bodies) may result from normal or excessive grooming behavior and tend to lodge in the stomach.27 Although vomiting is not as frequent in ferrets with gastrointestinal (GI) foreign bodies as in other domestic species,27 clinical signs in ferrets with foreign-body obstruction are similar to those of other small animal patients with GI obstruction. These signs include diarrhea, anorexia, lethargy, bruxism, and pawing at the mouth. Foreign bodies in the intestinal tract may also be discovered during exploratory laparotomy in ferrets that have no clinical signs attributable to intestinal obstruction. Ferrets presented with intestinal foreign bodies are commonly less than 18 months of age.20
Esophageal foreign bodies are rare and should be treated as recommended in other small animal species. If possible, remove the foreign body by endoscopy or by advancing it into the stomach for retrieval by gastrotomy. An esophagotomy may be performed through a right lateral thoracotomy, median sternotomy, or midline cervical approach, but this procedure can be complicated by postoperative leakage or stricture formation.9
Most intestinal foreign bodies can be retrieved during an exploratory laparotomy. The ferret’s intestine lacks a distinct ileum; instead, the jejunoileum extends from the duodenojejunal flexure to the ascending colon.16 Evaluate the entire intestinal tract, as multiple foreign bodies may be present, and perform gastrotomy, enterotomy, or resection and anastamosis as in other small animals. Because the ferret’s intestinal tissue is very thin and fragile, gentle tissue handling is needed to prevent iatrogenic damage to the intestines. Isolate the intestinal surgery site from the abdominal cavity with moistened sponges.
For a gastrotomy, stabilize the stomach with full-thickness stay sutures of 4-0 nylon before making an incision (Fig. 11-3). Close the gastrotomy incision in one or two layers by using a simple interrupted or continuous suture pattern with 4-0 or 5-0 monofilament absorbable suture.
Liver Biopsy
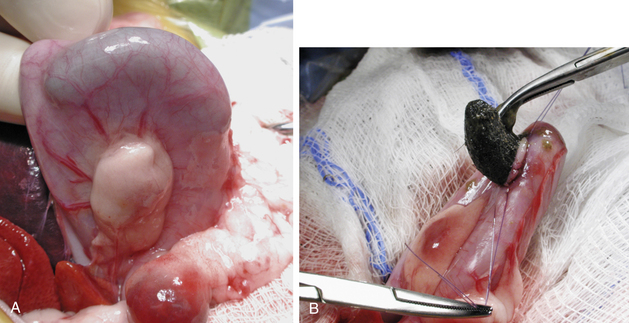
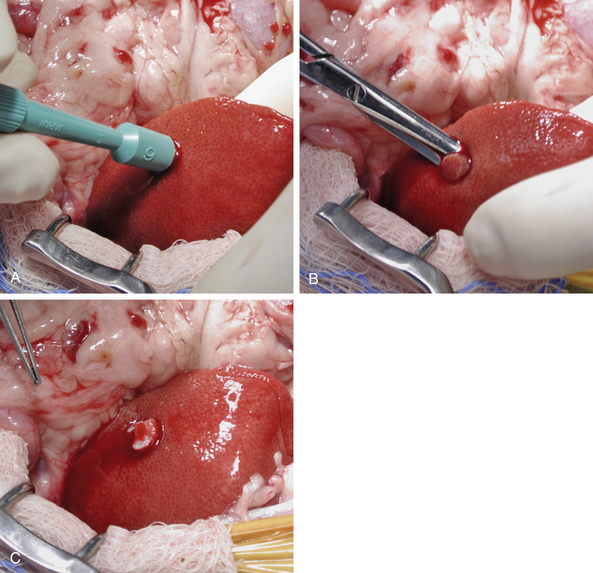
Ultrasound-guided fine-needle aspirates or biopsies are most commonly performed in ferrets to diagnose suspected liver disease. However, a liver biopsy may be performed during an exploratory laparotomy to help diagnose suspected or unsuspected liver disease such as hepatic lipidosis, lymphosarcoma, cholangiohepatitis, or neoplasia. If all lobes have a similar appearance and palpate similarly, a random liver biopsy is indicated. If a section of liver is protruding, a guillotine suture can be used. For this technique, place a preformed encircling ligature of 4-0 monofilament absorbable suture material around the protruding section of liver. Tighten the ligature until it has crushed through the hepatic parenchyma (Fig. 11-4). After completing several throws in the knot, excise the sample 1 to 2 mm distal to the ligature with Metzenbaum scissors or a scalpel blade. Take care to avoid crushing the sample and destroying architecture with forceps in handling it.
If a specific area of the liver is of interest, the tissue sample can be obtained by the transfixion method or with a 6-mm skin biopsy punch. For the transfixion method, place a ligature through the liver lobe approximately 8 to 10 mm from its edge. Tighten the ligature to crush through the parenchyma of half of the desired biopsy specimen. Make an additional throw at a right angle to the first ligature, tightening the ligature to crush the parenchyma for the second half of the specimen. Remove the sample 1 to 2 mm distal to the crushed area with a scalpel blade or Metzenbaum scissors. If the area of interest does not lie near the edge of the liver lobe, a 6-mm biopsy punch can be used. Push the biopsy punch through the liver parenchyma in the desired area, making sure not to penetrate the opposite surface of the liver (Fig. 11-5). Use extra caution if the biopsy site is close to the hilum so that no more than one half of the thickness of the liver lobe is penetrated. Remove the biopsy punch and separate the biopsy sample from the liver with scissors, being careful not to crush the sample. If culture and sensitivity testing of a liver sample is warranted, it can be obtained at this time. Control bleeding either by filling the defect with gelatin sponge hemostatic material and applying digital pressure for 3 to 5 minutes or by suturing the liver capsule with a fine absorbable monofilament suture in a cruciate pattern.
Gallbladder Surgery
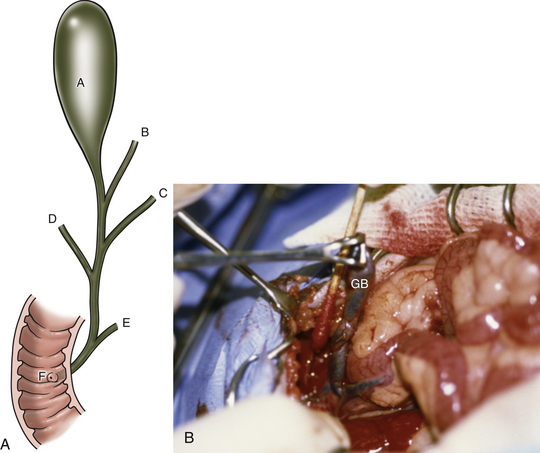
The biliary system of ferrets is similar to that of other domesticated animals and is composed of three hepatic ducts feeding into a common bile duct (Fig. 11-6). The gallbladder empties into the cystic duct, which joins the central hepatic duct to become the common bile duct. The main pancreatic duct joins the common bile duct before it empties into the duodenum at the major duodenal papilla.33
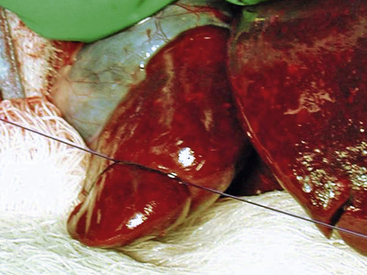
Choleliths can be located in the gallbladder, or they can obstruct the common bile duct as choledocholiths. Therefore thoroughly examine the biliary tree during exploratory abdominal surgery for gallstones. If the obstruction is located in the common bile duct, attempt to retropulse the stones by gently milking them backward into the gallbladder with your fingertips. Alternatively, make an incision in the antimesenteric border of the duodenum at the level of the major duodenal papilla. Cannulate the common bile duct with a 25-gauge catheter and flush the stones into the gallbladder; the duodenal papilla is generally located 2 to 3 cm distal to the cranial duodenal flexure. Once the stones are in the gallbladder, check the common bile duct for patency by flushing the duct with a 3.5-Fr catheter. If it is patent, perform a standard cholecystectomy (as in other domestic species) to prevent recurrent cholecystitis.24 Gently elevate the gallbladder from the hepatic surfaces of the right medial and quadrate liver lobes with sterile cotton-tipped applicators (see Fig. 11-6). Use hemostatic sponges on the liver surface to control hemorrhage. After dissecting the gallbladder and cystic duct to the level of the central hepatic duct, double-ligate the cystic duct with fine absorbable or nonabsorbable suture material or vascular clips and transect it. Submit sections of the gallbladder for bacterial culture and histopathologic examination and submit the gallstones for crystallographic analysis. To date, all ferrets with gallstones have had a histologic diagnosis of cholecystitis, with 1 of 4 ferrets having a positive bacterial culture. All choleliths have been composed primarily of bile pigment and bile salts.
Endocrine System
Surgery of the Adrenal Gland
Adrenal neoplasia is common in ferrets. Cortical hyperplasia, adenomas, and carcinomas secreting estrogen and other steroid hormones are the most common tumor types (see Chapter 7).4,28 Other types of adrenal gland neoplasia have been reported, although with a much lower frequency. These include spindle cell tumors, pheochromocytomas, teratomas, and myelolipomas.21
Adrenocortical disease in ferrets is not Cushing’s disease. Instead of secreting excess cortisol, affected adrenal glands produce estrogens and androgens.22,38,48 The most common clinical sign associated with adrenocortical disease is alopecia, which begins on the tail and progresses cranially along the dorsum, flanks, and body.19,49,50 Affected females often present with enlarged vulvas, and affected males may show sexual behavior, increased musky odor, or present with stranguria or urinary obstruction secondary to an enlarged prostate or paraurethral cysts.
Ferrets typically present with clinical signs after 3 years of age (range 1 to 7.5 years). One retrospective study found that adrenocortical disease occurred more commonly in females; however, others have found an equal distribution between the sexes.37,49,50 Most ferrets with adrenocortical disease have been neutered before 6 weeks of age; the disease occurs less commonly and at an older age in sexually intact ferrets.37 An association between adrenocortical disease in ferrets and neutering at an early age is suspected. This association is postulated to be caused by loss of negative gonadal feedback to the release of gonadotropin-releasing hormone (GnRH) from the hypothalamus, resulting in persistent stimulation of the adrenal cortex by luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Both FSH and LH receptors have been isolated in the adrenal glands of affected ferrets; results of one study in ferrets showed a correlation between age at neutering and age at onset of adrenocortical disease.41 Genetic factors are also potentially involved in predisposition to this disease (see Chapter 7). However, more studies are needed to prove a cause-and-effect relationship.
Adrenocortical disease in ferrets is often tentatively diagnosed by clinical signs. Dorsal symmetric bilateral alopecia with or without pruritus is a common presentation. Alternatively, hair loss may begin on the tail. Differential diagnoses for these presentations include seasonal hair loss, external parasites, extended estrus, ovarian remnants, or severe metabolic disturbances.4 Physical examination may reveal a mass palpable cranial to the left kidney. The cranial aspect of the right kidney is generally not accessible to palpation. Abdominal ultrasound is used to identify an enlarged adrenal gland or glands and to determine whether the disease is unilateral or bilateral. Color-flow Doppler can be used to determine the extent of caudal vena cava invasion when a right adrenal gland is affected. Ultrasound also screens for the presence of other disease processes in abdominal organs. The diagnosis can be confirmed with an adrenal hormone panel that measures plasma concentrations of 17-hydroxyprogesterone, androstenedione, and estradiol.38
Before surgery, a complete blood count and plasma biochemical analysis should be performed on each patient to evaluate overall health. Commonly, hypoglycemia is present because of concurrent insulinoma. Whether or not the ferret exhibits hypoglycemia, always palpate the pancreas intraoperatively for detection of insulinomas; if encountered, these should be resected (see “Pancreatic Surgery,” below).
Medical management for adrenal disease in ferrets is discussed in Chapter 7. If surgery is elected, the surgical approach is as follows:
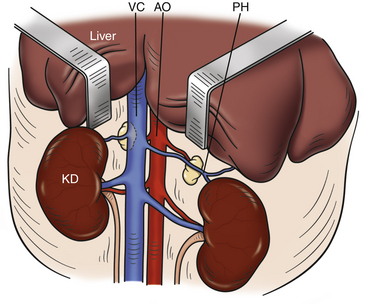
Make a ventral midline incision, extending it caudally from the xiphoid as needed to allow access to both adrenal glands. The left adrenal gland is often embedded in fat and is located cranial and medial to the left kidney (Fig. 11-7). The right adrenal gland lies craniomedial to the right kidney and adjacent to the caudal vena cava. Normal adrenal glands are whitish pink, 2 to 3 mm wide, and 6 to 8 mm long.28,30 Remember that not all diseased adrenal glands are enlarged, and the glands should always be palpated for abnormal texture. If the entire left adrenal gland is not visible, use mosquito hemostats and cotton-tipped applicators to carefully dissect the thin layer of peritoneum and fat surrounding the gland to free it. To expose the right adrenal gland, the hepatorenal ligament (which attaches the caudate lobe of the liver to the right kidney) must be incised. Yellow-brown discoloration, an overly firm or gritty texture, and the presence of cysts or enlargement are all indications of a diseased adrenal gland.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree