Chapter 77 Sodium Monofluoroacetate (1080)
Sodium monofluoroacetate (fluoroacetate, SMFA, and compound 1080) is a pesticide used to control rodents and other pests in the United States and worldwide. Commonly termed 1080, this compound was identified as an effective rodenticide in the 1940s. Fluoroacetamide (compound 1081) was developed soon afterward as a less toxic alternative to 1080.1 Both compounds are white, odorless, tasteless, water soluble, and highly toxic poisons.2 Fluoroacetate also occurs naturally in a variety of plants in Africa (Dichapetalum cymosum and Dichapetalum toxicarium), Australia (Acacia georginae, Gastrolobium spp and Oxylobium spp), and South America (Palicourea marcgravii).1 These and other plants containing fluoroacetate are known to poison livestock. Because these compounds are toxic to carnivores, they have been manufactured for the control of predators, such as the coyote (Canis latrans). At the present time, the use of 1080 is restricted to trained, licensed applicators. Although this restriction is intended to control its release in the environment and to target specific predators, unintentional poisoning of nontargeted animals occurs. In the United States the only currently registered use of 1080 is in a livestock protection collar for sheep and goats. There is no currently registered use of 1081 in the United States.3
Compound 1080 is also an important toxic metabolite associated with the chemotherapeutic anticancer agent 5-fluorouracil and some fluorinated ethanes that may be toxic when inhaled.4–6 Clinical signs associated with the adverse effects of 5-fluorouracil and fluorinated ethanes resemble those seen with 1080 toxicity.5,6
SOURCES
Compound 1080 is manufactured in the United States for use in livestock protection collars for sheep and goats to control predators. The Environmental Protection Agency has tightly restricted the use of compound 1080 in these collars. Uses of 1080 and 1081 in rodenticides have been canceled.3 Evidence suggests that unlicensed operators may use illegally acquired 1080 or 1081 to poison pests or predators.
Poisoning of pets with 1080 or 1081 may result from the ingestion of poisoned rodent carcasses, particularly when the gastrointestinal tract is consumed, because rodents require a relatively high dose of fluoroacetate to be killed. Alternatively, pets may be poisoned intentionally. In New Zealand large quantities of baits containing 0.08% or 0.15% of 1080 are sprayed aerially to control introduced pests, such as the rabbit and brushtail possum (Trichosurus vulpecula).7 Secondary poisoning of cats and dogs results from their ingestion of these dead or dying animals.
TOXICOKINETICS
Animals have different susceptibilities to 1080 (and 1081) poisoning. The reason for the wide variation in toxicity is not known. Birds are most resistant to poisoning, followed by (in order of increasing sensitivity) rodents, primates, horses, rabbits, ruminants, and carnivores. Because tissue residues are minimal, the ingestion of muscle from a poisoned carcass is unlikely to cause toxicity in carnivores. Compound 1081 has a slower onset of action, and animals may display fewer neurological signs.1 The toxic dose of 1080 for dogs or cats is as little as 0.05 mg/kg body weight (Table 77-1). The quantity of 1081 necessary to kill dogs, rabbits, and sheep is two to five times more than the 1080 dose.1
Table 77-1 Toxic Doses of 1080 in Select Species
| Species | Oral Lethal Dose (mg/kg body weight) | LD50 (mg/kg body weight) |
|---|---|---|
| Cat | 0.3–0.5 | 0.20 (IV) |
| Dog | 0.06–0.2 | 0.05–1 |
| Cattle, sheep, and goats | 0.15–0.7 | 0.25–0.5 |
| Horse | 0.5–2.75 | 0.35–0.55 |
| Possum | 0.3 | |
| Pigs | 0.3 | 0.4–1 |
| Prairie dogs (Cynomys ludovicianus) | 0.173 | |
| Rabbit | 0.8 | |
| Rodents (rats and mice) | 2–8 |
LD50, Median lethal dose; IV, intravenous.
MECHANISM OF TOXICITY
Compounds 1080 and 1081 are readily absorbed from the gastrointestinal and respiratory tracts, abraded skin, and mucous membranes, but not through intact skin. They are not known to accumulate in any one tissue.1
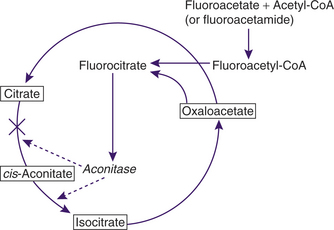
The classic theory of 1080 poisoning is the so-called lethal synthesis effect on the Krebs or tricarboxylic acid (TCA) cycle (Figure 77-1). Fluoroacetate and fluoroacetamide combine with acetyl coenzyme A (CoA) to form fluoroacetyl CoA, which then combines with oxaloacetate to produce fluorocitrate. Fluorocitrate inhibits aconitase and the oxidation of citric acid, resulting in blockage of the TCA cycle, energy depletion, citric and lactic acid accumulation, and a decrease in blood pH.2 The inhibition of aconitase interferes with cellular respiration and the metabolism of carbohydrates, fats, and proteins.1,2 Recent work presents evidence that fluorocitrate is converted to 4-hydroxy-trans-aconitate, which binds very tightly to aconitase, thereby inactivating it.8 The end result is an accumulation of citrate, which binds with serum calcium. The decrease in available ionized calcium has been shown to prolong the QT interval in electrocardiograms in cats.9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree