CHAPTER 10 Small Animal Spinal Cord Disease
NEUROLOGIC EXAMINATION
The complete neurologic examination is described in Chapter 20. The components referable to the spinal cord are reviewed here. There are five parts to the neurologic examination:
Careful examination of all of these is necessary to determine whether a lesion is confined to the spinal cord and at what level. Remember that, as described in Chapter 5, spinal reflexes require only the specific peripheral nerves and the spinal cord segments with which they connect, whereas postural reactions depend on the same components as the spinal reflexes plus the cranial projecting general proprioceptive (GP) pathways in the spinal cord white matter to the brainstem, cerebellum, and frontoparietal cerebrum and the caudal projecting upper motor neuron (UMN) pathways that return from the cerebrum and brainstem and comprise tracts in the white matter of the spinal cord that terminate in the cervical and lumbosacral intumescences. These postural reactions test the integrity of nearly the entire peripheral and central nervous systems. By themselves, postural reactions are relatively nonlocalizing for a lesion.
Gait
The clinical signs of UMN and GP dysfunction are described in Chapters 8 and 9, and it is emphasized that most gait abnormalities involving these systems reflect a combination of UMN (spastic paresis) and GP (ataxia) clinical signs because of their close anatomic relationship. It also is strongly urged that differentiation between the clinical signs caused by disruption of these two systems is of no practical value. With spinal cord lesions that affect the UMN and GP systems between spinal cord segments T3 and L3, there is a tendency to describe the pelvic limb gait as just ataxic or occasionally just paretic. In reality, the gait abnormality usually reflects a dysfunction of both systems and should be referred to as pelvic limb ataxia and paresis or paraparesis and pelvic limb ataxia. The same rule holds for cervical spinal cord lesions that occur between spinal cord segments C1 and C5: tetraparesis (quadriparesis) and ataxia of all four limbs. The terms paraplegia and tetraplegia should be used only when there is absolutely no voluntary movement in the pelvic limbs or in all four limbs, respectively. To observe ataxia, there has to be voluntary movement. Therefore, the term ataxia is inappropriate for a paraplegic or tetraplegic animal. Patients that are recumbent in the pelvic limbs (T3-L3 lesion) or in all four limbs (C1-C5 lesion) and show no voluntary movement when picked up and moved along the ground surface while suspended but exhibit some voluntary limb movement when recumbent should be described as nonambulatory paraparetic or tetraparetic. The same terminology applies to lesions that involve the lumbosacral or cervical intumescence, except that the quality of the paresis reflects a lower motor neuron (LMN) dysfunction.
A patient with lesions that affect the pontomedullary or spinal cord UMN and GP systems and is still able to walk unassisted exhibits a delay in the onset of protraction, which is the swing phase of the gait; a stiff quality of movement; and often a longer stride, due to a delay in the termination of protraction. In the thoracic limbs, this causes a floating, overreaching action, with the limb in extension. It is important to recognize the extension of the thoracic limb joints and the prolongation of the protraction, which causes the paw to be placed on the ground farther cranially than normal. This is in contrast to the thoracic limb gait in a cerebellar disorder, where the protraction is abrupt and all the limb joints flex, causing the limb to move more dorsally toward the neck. As protraction is completed, the limb is commonly misdirected. This difference in the thoracic limb gait between a UMN-GP system dysfunction and a cerebellar dysfunction is difficult to describe but should be obvious when you study the videos of these disorders. (See Chapter 13 for a description of the gait of a cerebellar disorder.) In UMN-GP system disorders, on protraction, the affected limb may abduct excessively, especially on turns, when it is often referred to as circumduction. The limb may also adduct excessively before it supports weight. This may appear as a crossing of the limbs as they are advanced. Often, the dorsal surface of the paw will scuff the ground on protraction or the patient will support its weight on the dorsal surface of the paw. Occasionally a pelvic limb is flexed excessively on protraction, creating a hypermetria in the gait. The trunk may also appear to be unstable and to sway as the patient walks, especially on turns. Remember that these clinical signs reflect dysfunction in both the UMN and the GP systems. There is ample opportunity in the disease section of this chapter to visualize on videos what is described here.
Many clinicians use a grading system to assess the degree of pelvic limb function as an aid in determining the prognosis and to evaluate response to therapy. This was originally designed for dogs with thoracolumbar spinal cord injury resulting from intervertebral disk extrusions. This is a common disorder in dogs in which numerous surgical procedures have been performed over many decades. However, it is applicable to any spinal cord lesion at any level. Grade 0 refers to a patient with no voluntary movement in the pelvic limbs and therefore is paraplegic. Grade 5 refers to a patient with normal pelvic limb function. Patients with functional grades 1 and 2 are unable to stand in the pelvic limbs without assistance. When you hold the patient up by grasping the base of the tail, and only very slight movements of the pelvic limbs occur, this patient would receive a grade of 1 for its function. If voluntary movements readily occur but are delayed, awkward, and poorly placed, the degree of function would be grade 2. A patient with grade 3 function is able to stand up in its pelvic limbs without assistance but has great difficulty and is able to walk but with significant paresis and ataxia. A patient with grade 4 function readily stands up unassisted and exhibits only mild paresis and ataxia in its gait. These are grades of function, not grades of paresis and ataxia. When we describe a grading system that is used for horses (see Chapter 11), it refers to grades of dysfunction and is used primarily for horses with cervical spinal cord disease.
Postural Reactions
You were introduced to postural reactions in Chapter 5 as they relate to examining patients with neuromuscular disorders. It is critical to remember that postural reactions essentially require that all components of the peripheral nervous system and the central nervous system (CNS) that affect the limb you are testing be intact.
There is no test for just the UMN or just the GP system. Although a number of these postural reactions are described in the neurologic literature, in our experience the most reliable postural reaction is the patient’s ability to hop laterally on one limb. After that would be the paw replacement test. Hemiwalking is most useful when your patient is too large for you to be comfortable evaluating the hopping responses. Placing of the limbs is useful only in the small-sized patients and when the hopping responses are equivocal.
Spinal Reflexes
Flex and extend each limb to further appreciate the degree of muscle tone that you already assessed in the standing position and during the testing of the postural reactions. The only tendon reflex that I (AD) perform is the patellar reflex, which is an assessment of the femoral nerve and spinal cord segments L4, L5, and L6. The detailed anatomy of these tendon reflexes is described in Chapter 5. These tests should be performed before testing the flexor reflexes, in which a noxious stimulus is used. Test the flexor reflexes using a pair of forceps for compressing the skin at the base of the digits. Initially, exert just enough compression to obtain a flexor response or evidence of nociception. These flexor reflexes test various components of the spinal cord segments that comprise the lumbosacral or cervical intumescence and the related peripheral nerves that supply the limb being tested. The detailed anatomy of these reflexes is described in Chapters 5 and 9. Remember that these flexor reflexes test not only the reflex arc but also determine the integrity of the nociceptive pathway to the somesthetic cerebral cortex. The latter is very important in evaluating the level of severe focal spinal cord lesions as well as in making a prognosis for those lesions. Be sure to test all of these reflexes with the patient in both left and right recumbency.
When this testing has been completed and the patient is returned to the standing position, perform the cutaneous trunci reflex. (You may see this reflex referred to as the panniculus reflex, but that is a misnomer because the panniculus adiposus is a layer of fat in the trunk region and is not responsible for this cutaneous reflex; see Fig. 5-9.) Using your forceps, gently probe or squeeze the skin along the dorsal midline, starting at the level of the pelvis, and repeat this stimulus over each vertebra until you observe the cutaneous trunci contracting on both sides. In many normal small animals this may not occur until about the midlumbar region, and in a very few normal animals, it will not occur at all. The forceps pressure stimulates impulses in the dorsal branches of the spinal nerves that supply the area stimulated. Because of the short caudal distribution of these dorsal branches, each spinal nerve supplies the skin for a distance of about two vertebrae caudal to the intervertebral foramen, where the spinal nerve emerges from the vertebral canal. The general somatic afferent (GSA) neurons that are stimulated synapse in the respective dorsal gray column on long interneurons whose axons enter the adjacent fasciculus proprius bilaterally but predominantly on the contralateral side, in our opinion. Here these axons course cranially to the C8 and T1 spinal cord segments to terminate in the ventral gray columns by synapsing on the general somatic efferent (GSE) neurons that innervate the cutaneous trunci via the brachial plexus and the lateral thoracic nerve. Recall that this reflex is lost in injuries that cause avulsion of the roots of the spinal nerves that supply the brachial plexus. In these patients, compression of the skin at any level along the trunk elicits only a cutaneous trunci reflex on the side that is opposite from the affected thoracic limb. This reflex is also helpful in determining the level and prognosis of a severe transverse spinal cord lesion such as an injury resulting from a vertebral column fracture or an intervertebral disk extrusion. Similar to nociception, it requires a bilateral transverse lesion to interfere with the pathway of these long interneurons. This cutaneous reflex is especially helpful in patients that are very stoic and respond poorly to most any noxious stimulus to any part of their body.
Cranial Nerves
The examination of cranial nerves is described in Chapters 6 and 7. When presented with a patient that exhibits clinical signs of spinal cord disease, the cranial nerve examination is important for determining whether the spinal cord signs are part of a multifocal disease process. It is also important to determine whether the spinal cord lesion is at a level where it can interfere with the sympathetic pathway to the head and cause Horner syndrome. That syndrome is observed when the cranial nerves are examined.
SUMMARY OF POSSIBLE CLINICAL SIGNS RELATED TO SPINAL CORD LESIONS
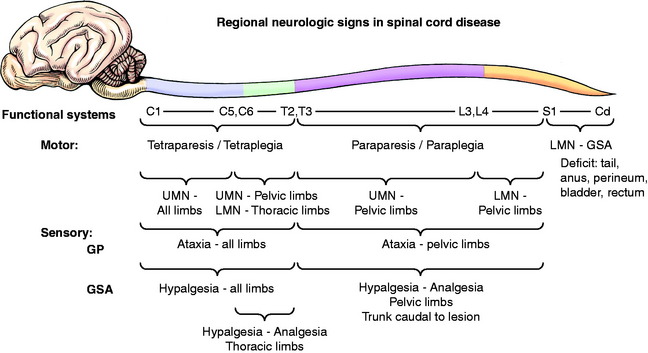
The spinal cord is divided into four regions on the basis of the clinical signs that are exhibited when any one of these four regions is affected (Fig. 10-1).
Lumbosacral: Fourth Lumbar to Fifth Caudal Spinal Cord Segments
Thoracolumbar: Third Thoracic to Third Lumbar Spinal Cord Segments
Caudocervical: Sixth Cervical to Second Thoracic Spinal Cord Segments
Craniocervical: First Cervical to Fifth Cervical Spinal Cord Segments
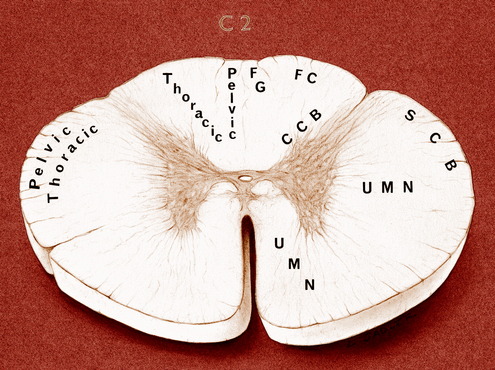
Most compressive lesions that affect the cranial cervical spinal cord segments cause more obvious clinical signs in the pelvic limbs than in the thoracic limbs. Explanations for this include the following: lesions that compress from the periphery of the spinal cord affect primarily the superficial tracts, which contain cranially coursing GP pathways from the pelvic limbs (Fig. 10-2); the pelvic limbs are further removed from the center of gravity, which is just caudal to the thoracic limbs; more UMN pathways terminate in the cervical intumescence than in the lumbosacral intumescence. Occasionally a cervical spinal cord lesion causes more obvious clinical signs in the thoracic limbs. This is seen with caudocervical lesions that affect the gray matter in the intumescence, causing LMN signs in the thoracic limbs. With more craniocervical lesions, this occurs when the spinal cord lesion affects the tracts closer to the gray matter and spares the more superficial tracts. These are predominantly UMN pathways to the cervical intumescence. A glial neoplasm that arises in the gray matter and grows peripherally toward the surface of the spinal cord will do this as will a midline intervertebral disk protrusion that compresses the spinal cord dorsally and laterally, making the spinal cord form a tent shape over the compressing mass. This disparity in clinical signs is also seen in many dogs with an atlantoaxial subluxation.
Bladder and occasionally rectal dysfunction commonly accompanies spinal cord lesions. These lesions were described, along with the anatomy involved, in Chapter 7. Lesions in the sacral segments result in LMN incontinence in urine and feces; UMN incontinence is common with severe lesions of the cervical and thoracolumbar spinal cord segments. This incontinence is a primary concern in your treatment of the patient.
Schiff-Sherrington Syndrome and Spinal Shock
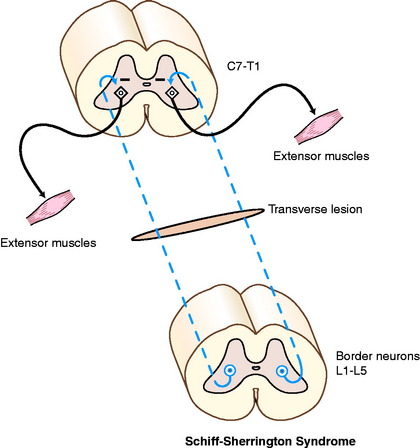
These two separate clinical disorders often present at the same time in a patient and appear to be exceptions to the rules that we have established for UMN and LMN disorders. They occur only with peracute, usually transverse, spinal cord lesions between the T3 and L3 spinal cord segments. Fractures of the vertebral column are the most common cause of such lesions. Others include infarction caused by fibrocartilaginous emboli and the myelopathy associated with peracute intervertebral disk extrusions. These patients exhibit persistent severe extension of the thoracic limbs in most postures because of disinhibition of the extensor motor neurons in the cervical intumescence. However, when these patients are held up, the thoracic limb gait is normal except for a mild stiffness. These clinical signs represent the Schiff-Sherrington syndrome.79 The disinhibition is not the result of dysfunction in a UMN pathway, which is why these patients can walk so well with the thoracic limbs when the trunk and pelvic limbs are supported. The disinhibition is the result of a sudden loss of the axons in a long interneuronal pathway that originates from neuronal cell bodies primarily in the gray matter of the L1 to L5 spinal cord segments. These interneurons are referred to as border cells because they are located in the dorsolateral border of the ventral gray column of the lumbar spinal cord segments.89 Their axons course cranially in the fasciculus proprius and terminate by synapsing on thoracic limb extensor LMNs in the cervical intumescence (Fig. 10-3). Their normal function is to inhibit these extensor motor neurons. This extensor release phenomenon is observed only with peracute severe lesions, and it spontaneously resolves in about 10 to 14 days. The presence of the Schiff-Sherrington syndrome indicates a severe lesion and a guarded prognosis but does not indicate that recovery cannot occur.
The severe peracute thoracolumbar spinal cord lesion that is responsible for the Schiff-Sherrington syndrome usually causes paraplegia because of the complete interruption in function of the UMN pathways, and it usually causes analgesia caudal to the lesion because of interruption of the nociceptive pathways. As a rule, with progressive transverse spinal cord lesions, paraplegia occurs before analgesia, suggesting that the nociceptive pathways are the most resistant to spinal cord compression and ischemia. However, in these severe peracute transverse thoracolumbar spinal cord lesions in which the Schiff-Sherrington syndrome is present, there usually is severe pelvic limb hypotonia. If you examine this patient within a few hours of the onset of the paraplegia, there may be absent or very depressed pelvic limb tone and spinal reflexes. These paradoxical LMN-like pelvic limb signs in a patient with a UMN pathway interruption represent what is called spinal shock.88 In primates, spinal shock causes areflexia and atonia for 2 to 3 weeks. In domestic animals, the areflexia is observed for only a few hours after the onset of the lesion, but the hypotonia persists for 10 to 14 days, when it is replaced by normal tone at first and then by hypertonia. The reasons a UMN lesion causes LMN signs are poorly understood. One explanation is that the sudden loss of the UMN synapses, indirectly via interneurons or directly on the dendritic zones or the cell bodies of the alpha motor neurons, causes such a disruption to that LMN cell body that it cannot function for a variable period of time. In primates, more of these UMN pyramidal system synapses are directly on the LMN, which may explain the difference in reaction among species that is observed here. Some studies have found an excessive accumulation of the inhibitory neurotransmitter glycine in the lumbosacral intumescence in these patients.86 The basis for the release of glycine is unknown. It is important to understand this unique combination of clinical signs that results from a focal lesion in the UMN and GP systems and not make an anatomic diagnosis of a multifocal disorder.
In a recumbent patient that has a fracture and should not be manipulated, the basis for these clinical signs can be determined through minimal handling of the patient. With the patient lying on the floor, a table, or a stretcher, provide a rigorous noxious stimulus to a digit of a pelvic limb; no movement occurs or there is just a mild reflex flexion if it is a few hours after the onset of clinical signs. Note the bilateral pelvic limb hypotonia. Provide very minimal compression of a digit of one of the hyperextended thoracic limbs or just a light squeeze of that forepaw with your hand, and note the immediate vigorous voluntary withdrawal of the limb. This tells you that you have a transverse thoracolumbar spinal cord lesion with Schiff-Sherrington syndrome in the thoracic limbs and spinal shock in the pelvic limbs. By providing forceps compression of the skin on the midline of the back as a noxious stimulus, starting in the caudal lumbar region and progressing cranially, a line of analgesia and/or a cutaneous trunci reflex can be found that locates the focal lesion, and imaging studies can be pursued with no further manipulation of the patient. If the patient does not have a fracture, its ability to walk with the thoracic limbs when supported differentiates the Schiff-Sherrington syndrome from a severe cervical spinal cord lesion.
(For a complete description of disorders that affect the lumbosacral spinal cord segments or the peripheral nerves associated with these segments, see the case examples of neuromuscular disease in Chapter 5.)
SMALL ANIMAL THORACOLUMBAR SPINAL CORD DISEASES
Signalment: 8-year-old spayed female boxer, Brittney
Chief Complaint: Unable to use the pelvic limbs
Anatomic Diagnosis: L4 to S1 spinal cord segments, worse on the left side
FIBROCARTILAGINOUS EMBOLIC MYELOPATHY
FCEM is a spinal cord lesion that is common in dogs but is uncommon in other species of domestic animals.* It is most common in young adult dogs of the larger breeds but it can occur as young as 3 months of age and it is common in the miniature schnauzer, Labrador retriever, and boxer breeds, in our experience. The clinical signs are peracute in onset and usually stabilize within 24 hours. Rarely, clinical signs may progress for 48 hours. Following that, there is no further progression or there is improvement, depending on the degree of ischemia or infarction that has occurred. The source of the fibrocartilage is assumed to be the intervertebral disk that is undergoing degeneration. This embolic fibrocartilage has the same collagen type that is found in the nucleus pulposus. How this degenerate fibrocartilage gains access to the spinal cord vasculature remains speculative. These emboli are more common in small arteries but also can be found in veins. Arteriovenous anastomoses do occur in the blood supply of the spinal cord and have been implicated in the distribution of the emboli. Protrusion of degenerate disk material into the adjacent ventral internal vertebral venous plexus has occasionally been observed at necropsy. It has been suggested that the normally avascular intervertebral disk is invaded by new growth of arteries when degeneration occurs in the annulus fibrosis and this is a route for these emboli to enter the arterial vasculature. We find this mechanism difficult to accept. In humans, degenerate intervertebral disk material can protrude into the adjacent vertebral body where there is ready access to the blood vessels in the marrow of the vertebra. One route of venous drainage from this marrow is into the ventral internal vertebral venous plexus within the vertebral canal. These intramedullary protrusions are referred to as Schmorl nodes. They are rare, or at least rarely identified in dogs, which may be because of dogs’ quadruped posture and the thick layer of cortical bone that is adjacent to the intervertebral disk. Reverse venous blood flow may be involved in the distribution of these emboli. Whenever an animal strains by contracting its trunk muscles with the glottis closed, the increased pressure in the thorax and abdomen interferes with the venous return to the heart and forces the venous blood into the vertebral venous plexus. This is the Valsalva maneuver, and it may play a role in the ability of these emboli to gain access to the spinal cord vasculature. The involvement of the intervertebral disk as the source of these emboli is also supported by the observation that these lesions occur primarily in the spinal cord. One report of brainstem lesions with fibrocartilaginous emboli indicated a possible source of emboli from cervical intervertebral disks.5 Magnetic resonance (MR) imaging often shows intervertebral disk degeneration at the level of the FCEM lesion in the spinal cord. These FCEM lesions can be unilateral or bilateral at any level of the spinal cord, and they affect various combinations of the gray and white matter. The lesions are usually limited to a few adjacent spinal cord segments. There are many examples in the following case examples that involve the various regions of the spinal cord. Caudal brainstem signs are rare and probably are associated with emboli arising from the cervical intervertebral disks.
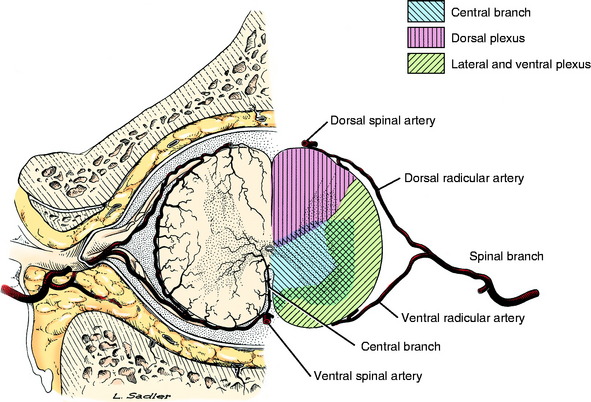
Because of the extensive collateral circulation to the spinal cord (Fig. 10-4), multiple blood vessels must be compromised to cause the degree of infarction and severe clinical signs seen in dogs similar to Brittney. This suggests that a sudden shower of emboli must occur at one time. At necropsy, these emboli can be found in many blood vessels in or near the lesions. Usually, this shower affects the blood vessels to a few adjacent spinal cord segments and the associated lesions often are scattered and asymmetric within these segments. Thus, the clinical signs are usually focal and often asymmetric, as seen in Brittney. FCEM may be much more common than we realize and not be extensive enough to cause clinical signs or cause only transient clinical signs. Most veterinarians have had the experience of being called by a distraught owner who has just found their pet dog collapsed and unable to stand, but by the time the dog arrives at the hospital for examination, the dog is walking normally. We believe that some of these transient episodes of collapse may be due to transient spinal cord ischemia caused by FCEM.
The three primary disorders that can cause an acute onset of relatively nonprogressive spinal cord dysfunction are external injury by objects in the environment, most commonly vehicles; internal injury resulting from intervertebral disk extrusions; and vascular compromise resulting from FCEM. The history usually permits substantiation or exclusion of external injury. Lacking that, vertebral column radiographs should provide that answer. To differentiate between the other two causes of these clinical signs, evidence of discomfort by the patient is more suggestive of an intervertebral disk extrusion than of FCEM, but exceptions are common for both of these disorders. Ultimately, immediate imaging is necessary because a diagnosis of an intervertebral disk extrusion usually requires emergency surgery. Myelograms in dogs with FCEM are helpful only in the small percentage of dogs in which intramedullary swelling is extensive. MR imaging is much more reliable in detecting the spinal cord edema that accompanies the ischemia or infarction caused by the fibrocartilaginous emboli.25 Be aware that MR imaging that is done in the first 24 to 48 hours after the embolic shower occurs may occasionally be normal.
Signalment: 3-year-old female Labrador retriever, Brandy
Chief Complaint: Unable to stand in the pelvic limbs
Differential Diagnosis: FCEM, intervertebral disk extrusion, neoplasia
The following videos show two more examples of the Schiff-Sherrington syndrome.
Video 10-3 shows Spanky, an 11-year-old male mixed-breed dog that was riding in a car that skidded on ice and struck a guard rail. Radiographs (shown in the video) revealed a fracture in the spinous process of the T6 vertebra and a collapsed intervertebral disk space between the T5 and T6 vertebrae, with no displacement. The video was made 4 days after the injury. Note the pelvic limb hypertonia and hyperreflexia and therefore no evidence of any spinal shock. Note the presence of nociception in the pelvic limbs, indicating that the spinal cord lesion has not caused a complete transverse dysfunction. A body cast was applied and Spanky regained the ability to walk with his pelvic limbs in about 5 weeks.72
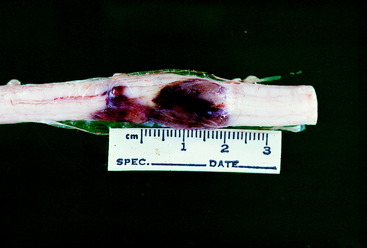
Video 10-5 shows Poison, an 8-year-old castrated male domestic shorthair that was struck by a vehicle 2 days before the video was made. Note the crossed extension in the pelvic limbs and the pelvic limb flexion when the tail was compressed with forceps. The latter is referred to as a mass reflex, which is another manifestation of hyperreflexia. Radiographs (see the video) showed a fracture at the articulation between the L3 and L4 vertebrae, with at least 50% displacement. Poison was euthanized and the necropsy showed that at the site of the fracture and displacement, there was only a sleeve of dura remaining. The parenchyma of the spinal cord at this site had been completely crushed and displaced into the adjacent spinal cord segments (Fig. 10-5).
Video 10-6 shows George, an adult Siamese cat that was found beside the road unable to use his pelvic limbs. Radiographs (see the video) showed a fracture at the articulation between the T13 and L1 vertebrae, with slight displacement. Note the pelvic limb flexion when the tail was compressed with forceps. This is an example of a mass reflex. George was euthanized and necropsy revealed hemorrhage and necrosis that involved all of the transverse section of the spinal cord at the level of the fracture (Fig. 10-6).
The spinal cord lesions resulting from injury caused by external trauma include the physical disruption of the high-risk spinal cord parenchyma as compared with the more resistant dural layer of meninges. See Fig. 10-6, which shows this severe spinal cord lesion. A contused spinal cord exhibits hemorrhage, edema, and necrosis due to compromise of the spinal cord’s vasculature. Following the traumatic event, there is continued degeneration of the injured spinal cord that progresses for a few hours, usually less than 24 hours. The basis for this is the subject of intense research directed at determining ways to treat these patients to prevent this progressive spinal cord destruction. Areas of interest include the release of neurotransmitters, such as toxic levels of glutamate, excessive accumulation of calcium ions, free radical species, nitrous oxide, and the release of various amines that cause vasoconstriction and subsequent ischemia and infarction.
Monoplegia
Signalment: 3-year-old castrated male malamute, Drake
Chief Complaint: Unable to use the left pelvic limb
Anatomic Diagnosis: A focal lesion on the left side, between the T3 and L3 spinal cord segments
Do not confuse this anatomic diagnosis with sciatic nerve paralysis, which looks much different. Review Video 5-44 of the collie dog Keane, described with Case Example 5-15; Keane has a left sciatic nerve injury due to a pelvic fracture. Dogs with sciatic nerve deficits can always advance the limb by hip flexion, and there is hypotonia of the crural muscles.
Differential Diagnosis: FCEM, intervertebral disk extrusion, neoplasia
Signalment: A 6-year-old castrated male Labrador retriever, Penfield
Chief Complaint: Unable to use the right pelvic limb
Examination: This examination took place the day after the onset of the clinical signs, which had not changed. Video 10-9 shows that this dog’s clinical signs are the mirror image of those observed in Drake in Case Example 10-3. Penfield exhibits a spastic monoplegia of his right pelvic limb.
Differential Diagnosis: The diagnosis is the same as that described for Drake in Case Example 10-3. The indication of discomfort when the initial clinical signs occurred is suggestive of a possible intervertebral disk extrusion. Radiographs and a myelogram diagnosed a unilateral right-side intervertebral disk extrusion between the L3 and L4 vertebrae. This was removed via a hemilaminectomy, and Penfield was walking with the right pelvic limb after about 3 weeks.
The following two videos show the same two clinical disorders that are described in this case example and Case Example 10-3.
Video 10-10 shows Dude, a 3-year-old male miniature schnauzer that had been outdoors unconfined during the night and was found in the morning unable to use his left pelvic limb. From your study of this video you should make the anatomic diagnosis of a focal spinal cord lesion on the left side between the T3 and L3 spinal cord segments. The differential diagnosis is the same as in this case example and Case Example 10-3 but must include external injury because the history cannot rule it out. The absence of any reluctance to try to walk as well as of any discomfort when palpated and the presence of extensive unilateral signs all suggest that external injury is unlikely. The young age of this dog suggests that a unilateral intervertebral disk extrusion is also less likely. In addition, this breed is at some risk for the development of fibrocartilaginous emboli. Radiographs and a myelogram were normal, which made FCEM the most presumptive diagnosis. Within 2 to 3 weeks, without specific therapy, Dude was walking well in the left pelvic limb. Figure 10-7 shows the kind of FCEM lesion that explains these clinical signs but from which recovery would not occur.
Video 10-11 shows Beaver Dam, an 8-year-old spayed female mixed-breed dog that was presented because of difficulty using the left pelvic limb. This dysfunction began when the dog fell down a flight of stairs and had not changed significantly since that time. From your study of the video, you should make the same anatomic and differential diagnosis as for Dude in the previous video. The video of Beaver Dam shows the radiographs, myelogram, and CT imaging, which diagnosed intervertebral disk extrusions at the L1-L2, and L3-L4 vertebral articulations. The owner elected to treat the dog medically, and no follow-up on its success is available.
Paraparesis and Ataxia
Signalment: 5-month-old male Labrador retriever, Cassidy
Chief Complaint: Unable to walk normally with the pelvic limbs
Although we tend to relegate neoplasia to older dogs, there are many exceptions to this, and one of them occurs at this location in dogs. Nephroblastoma is a unique intradural, extramedullary (extraaxial) neoplasm that occurs in young dogs, usually between spinal cord segments T10 and L2 (Figs. 10-8 through 10-10).91 Most of our experience and reports in the literature involve dogs younger than 3.5 years of age, many of them only a few months old. There are numerous reports, mostly in the European literature, that describe this neoplasm as an ependymoma.58,92,109 In the first two editions of this text, I (AD) recognized that this was not intramedullary and therefore was not an ependymoma, and I called it a neuroepithelioma based on the morphology of the cells and the abundance of tubular elements in the neoplasm. Since then, we have recognized tubular structures that resemble renal glomeruli and have diagnosed this neoplasm as a presumptive nephroblastoma. It is an embryonic neoplasm that may arise from mesonephric tubules. This is supported by the positive immunocytochemical staining of the neoplastic cells for a polysialic acid marker of this tumor; the test was developed in children by Dr. J. Roth in Switzerland.78
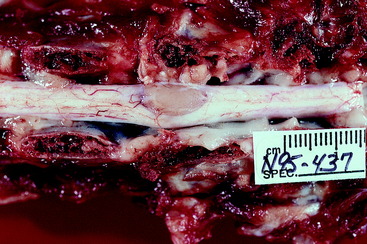
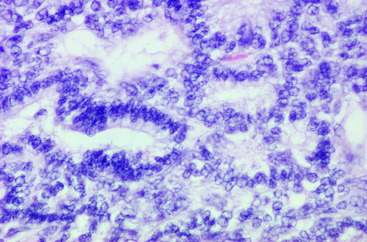
Figure 10-8 A nephroblastoma in a 5-month-old Labrador retriever at the level of the T13 spinal cord segment.
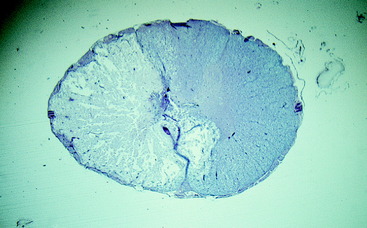
Figure 10-10 A transverse section of the nephroblastoma in Fig. 10-9. The nephroblastoma is intradural but extramedullary. Note the marked spinal cord compression.
In children, this nephroblastoma occurs in the kidney and is called a Wilm tumor. A gene located on chromosome 11 has been identified for this tumor in children. Using antibodies developed for the protein product of this tumor gene, immunocytochemical staining has identified this protein product in the canine neoplasm.76 The unique location of this neoplasm between spinal cord segments T10 and L2 correlates with the site of embryonic renal development from intermediate mesoderm. This is adjacent to the development of the somitic sclerotomes that envelope the neural tube that forms the thoracolumbar spinal cord. A Wilm tumor has three components, sheets of unorganized epithelial cells referred to as blastemal cells; tubular elements lined by epithelial cells that vary from squamous to cuboidal and some of which form glomerular structures; and a fibrous component that consists primarily of bundles of collagen. The canine neoplasm is made up primarily of the first two components (Figs. 10-11 through 10-15). A nephroblastoma in this spinal cord location is very rare in children. In dogs, this neoplasm does occur in the kidney but it is much more common adjacent to the spinal cord. There are no reports of this neoplasm being in both locations in the same patient.
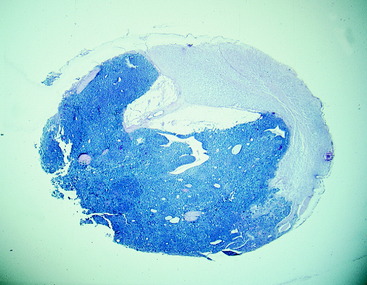
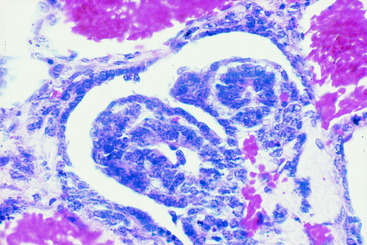
Figure 10-12 A microscopic section of the nephroblastoma in the dog in Fig. 10-11. The nephroblastoma is growing in the subarachnoid space, compressing the spinal cord to the right side.
Typically, the clinical signs occur in one pelvic limb prior to their occurrence in the other, which reflects the asymmetric location of the neoplasm. We suspect this is a very slowly growing neoplasm and recognize that the spinal cord can be slowly compressed by an extramedullary mass for a long time before the autoregulation of spinal cord vascular perfusion fails and clinical signs occur. Therefore, when these clinical signs occur, be aware that the spinal cord is already very compressed, and if surgery is to be considered it should be done as soon as possible. Often at necropsy, the spinal cord consists only of a thin 1- to 3-mm quarter-moon-shaped shell covering one side of the neoplasm (see Figs. 10-10 through 10-12). When surgeons remove this neoplasm they often see the severely compressed spinal cord slowly start to fill the space where the mass was removed. Postoperative radiation therapy should be done to avoid recurrence of the neoplasm.
Vertebral malformation with kyphosis and secondary spinal cord compression is a realistic consideration in this dog. This vertebral malformation is usually in the midthoracic portion of the vertebral column.49,67 Although these dogs have the vertebral malformation at birth, the clinical signs of spinal cord compression usually do not occur until a few months of age but prior to 1 year of age. We believe that the kyphosis that causes the spinal cord compression develops at the site of the malformation as the dog grows, and this accounts for the age of onset of the progressive spastic paraparesis and ataxia of the pelvic limbs. Occasionally, on your physical examination of the patient, you can see and palpate the kyphosis. However, it is easy to overlook if you do not take the time to carefully examine the patient for it. This malformation is readily seen on radiographs. It was not visible or palpated in Cassidy. (See Videos 10-15 and 10-16.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree