CHAPTER 8 Skin Immune System and Allergic Skin Diseases
Introduction
The subject of immunodermatology has seen a tremendous emergence of new discoveries, findings, and laboratory techniques. An adequate review of this information is beyond the scope of this chapter. For the practitioner, student, and academician interested in details, numerous texts on immunology and immunodermatology are available, and two are particularly relevant to the horse.5,8 This section is confined to a brief overview of the concepts regarding immunology of the skin. To comprehend this discussion or to read the current scientific literature on many cutaneous diseases, the reader has to understand some newer terminology about cell-surface antigens, cytokines, and adhesion molecules, which are basic components of all immunologic discussions.
Cluster differentiation antigens
The understanding of current literature involving immune responses requires an understanding of cell-surface determinants, which are referred to by the cluster differentiation (CD) nomenclature (Table 8-1). This nomenclature is applied to antigens that have been detected on the surface of cells with monoclonal antibodies, and these are usually assigned a number. Initially these antigens were studied and shown to be specific to or limited to a specific group of cell types, which allowed identification of types of cells present in tissues or exudates. Further studies have allowed for the recognition of the function and, in many cases, the structure of these antigens. It is now known that many of the surface molecules are various immunoglobulins (Igs), carbohydrates, enzymes, adhesion molecules to bind with other cells, and receptors for the various Igs and chemicals (cytokines) secreted by cells to communicate with surrounding cells.
TABLE 8-1 Glossary of Cluster Differentiation Antigens
| Antigen | Comment |
|---|---|
| CD1 (a, b, c) | Molecules are markers of dendritic antigen-presenting cells (Langerhans cells, dermal dendritic cells) |
| CD3 | The T-cell receptor/CD3 complex is only expressed on the surface of mature T-cells |
| CD4 | Expressed by MHC class II-restricted T-helper cells. Macrophages and dendritic antigen-presenting cells can upregulate CD4 in some instances |
| CD5 | Expressed by almost all mature T-cells; a minor subset of B-cells can express CD5 (B1 cells) |
| CD8 | Expressed by MHC class I-restricted T suppressor/cytotoxic cells |
| CD11 (a, b, c) | The ß2 integrins (CD11/CD18) are the major adhesion molecule family of leukocytes. Most leukocytes express one or more members of this family. CD18 is the ß2 subunit that pairs with one of four α subunits to form a heterodimer. The three α subunits are: CD11a (all leukocytes), CD11b (granulocytes, monocytes, some macrophages), CD11c (granulocytes, monocytes, dendritic antigen-presenting cells) |
| CD14 | Receptor for LPS (endotoxin) and LPS binding protein complexes. CD14 is expressed on monocytes, subsets of macrophages, subsets of B-cells |
| CD18 | The ß2 integrins (CD11/CD18) are the major adhesion molecule family of leukocytes. Most leukocytes express one or more members of this family. CD18 is the ß2 subunit that pairs with one of four α subunits to form a heterodimer. The three α subunits are: CD11a (all leukocytes), CD11b (granulocytes, monocytes, some macrophages), CD11c (granulocytes, monocytes, dendritic antigen-presenting cells) |
| CD21 | CD21 is expressed on mature B-cells |
| CD44 | A broadly expressed adhesion receptor (for hyaluronate) on many cell types; involved in lymphocyte trafficking and activation |
| CD45 | CD45 is the leukocyte common antigen family |
| CD49 | The β1 integrins are broadly expressed on leukocytes |
| CD50 | CD50 (ICAM-3) is broadly expressed by leukocytes |
| CD54 | Intracellular adhesion molecule (ICAM) family consists of at least four members. CD54 (ICAM-1) is broadly expressed (leukocytes, endothelium) and is a major ligand of CD11a. CD54 is upregulated on endothelium, leukocytes, and even on epithelium in inflammation (by inflammatory cytokines) and is important in leukocyte transmigration. Expression of CD54 on dendritic/APC enhances T-cell activation through CD11a |
| CD79 (a, b) | CD79a is expressed throughout all stages of B-cell development and persists into the plasma cell stage |
| CD90 | CD90 (Thy-1) is expressed by dermal dendritic cells |
Cytokines and Chemokines
Cytokines are secreted from cells and function in communicating with surrounding cells (Table 8-2). They are soluble proteins or glycoproteins that affect the growth, differentiation, function, and activation functions of other cells. These soluble hormonelike molecules were initially discovered in association with lymphocytes and termed lymphokines, or monocytes and termed monokines. As it was discovered that many other cells produced the same substances, these terms, and others such as secretory regulins and peptide regulatory factors, were no longer considered appropriate. Cytokines are transiently produced and exert their biologic activities via specific cell-surface receptors of target cells, which may be expressed only after activation of the cell. Each mediator usually has multiple overlapping activities. Numerous cytokines have been described, and typically, they may perform several different functions, depending on the tissue they interact with and the other cytokines that may be present. In different environments, the same cytokine may even have opposite effects.
TABLE 8-2 Immunologic Properties of Cytokines
| Cytokine | Properties |
|---|---|
| Interleukins | |
| IL-1 | Immunoaugmentation (promotes IL-2, IFN-α, CSF production by T-cells); promotes B-cell activation (promotes IL-4, IL-5, IL-6, IL-7 production and immunoglobulin synthesis); stimulates macrophages and fibroblasts; induces arachidonate metabolism |
| IL-2 | Activates T and natural killer (NK) cells; promotes cell growth and immunoglobulin production; activates macrophages |
| IL-3 | Promotes growth of early myeloprogenitor cells, eosinophils, mast cells, and basophils |
| IL-4 | Promotes B-cell activation and IgE switch; promotes T-cell growth; synergistic with IL-3 for mast cell growth |
| IL-5 | Eosinophil growth; B-cell growth and chemotaxis; T-cell growth |
| IL-6 | Terminal differentiation factor for cells and polyclonal immunoglobulin production; enhances IL-4 induced IgE production; promotes T-cell proliferation and cytotoxicity; promotes NK cell activity; activates neutrophils |
| IL-7 | Lymphopoietin |
| IL-8 | Chemoattractant for neutrophils, T-lymphocyte, basophils; increases histamine release from basophils |
| IL-9 | Maturation of erythroid progenitor cell tumor growth; synergistic with IL-3 for mast cell growth |
| IL-10 | Downregulation (inhibits production of IL-1, IL-2, IL-4, IL-5, IL-5, IL-8, IL-12, TNF-α, IFN-γ, MHC class II expression) |
| IL-11 | Megakaryocyte, lymphocyte, and plasma cell growth |
| IL-12 | Cytotoxic lymphocyte maturation; NK cell activation and proliferation |
| IL-13 | Similar to IL-4; enhances production of MHC class II and integrins; reduced production of IL-1 and TNF; activation of eosinophils |
| IL-14 | Expands clones of B-cells and suppresses immunoglobulin secretion |
| IL-15 | Proliferation; increased cytotoxicity of T-cells, NK cells; expression of ICAM-3; B-cell growth and differentiation |
| IL-16 | Chemoattractant, growth factor |
| IL-17 | Autocrine proliferation and activation |
| IL-18 | Similar to IL-12; inhibits IgE production by increasing IFN-γ |
| Colony-Stimulating Factors | |
| Granulocyte CSF | Neutrophil growth |
| Monocyte CSF | Monocyte growth |
| Granulocyte-monocyte CSF | Monomyelocytic growth |
| Basic fibroblast growth factor (βFGF) | Fibroblast growth and matrix production |
| Platelet-derived growth factor | Proliferation; chemoattractant for fibroblasts; active in wound healing |
| Stem cell factor | Chemoattractant; with IL-3 stimulates growth; also has histamine-releasing activity |
| Transforming growth factor (TGF) | Inhibits IL-2-stimulated growth; switch factor for IgA but inhibits IgM and IgG production; counteracts IL-4 stimulation of IgE; inhibits cytotoxicity |
| Interferons | |
| IFN-α | Antiviral; antiproliferative; immunomodulating (activation of macrophages; proliferation of B-cells; stimulation of NK cells); inhibit fibroblasts |
| IFN-ß | Antiviral; antiproliferative; immunomodulating (activation of macrophages, proliferation of B-cells, stimulation of NK cells); inhibit fibroblasts |
| IFN-γ | Immunomodulation (activation of macrophages; proliferation of B-cells; stimulation of NK cells); antiproliferative; antiviral; inhibit fibroblasts; inhibits IL-4-mediated expression of IgE receptors and the IgE switch |
| Tumor Necrosis Factors | |
| TNF-α | Inflammatory, immunoenhancing, and tumoricidal |
| TNF-ß | Inflammatory, immunoenhancing, and tumoricidal |
Cytokines may affect the same cell in a permissive, inhibitor, additive, or suppressive manner. Cytokines are involved in virtually every facet of immunity and inflammation, including antigen presentation, bone marrow differentiation, cellular recruitment and activation, adhesion molecule expression, and acute-phase reactions (see Table 8-2). The particular cytokines produced in response to an immunologic insult will determine whether an immune response develops and whether the response will be humoral, cell-mediated, or allergic. Certain cell types, particularly T-lymphocytes, may secrete different patterns of cytokines, and this has been used to subclassify these cells and their associated different functions. A large group of cytokines have been identified that have as their sole or major purpose the direction of the movement of cells involved in inflammation and the immune response. These have been termed chemokines (Table 8-3).
TABLE 8-3 Chemokines and Their Actions
| Chemokine | Target Cells | Biological Effects |
|---|---|---|
| CXC (α) Family | ||
| BCA-1 (B-cell-attracting chemokine-1) | B-lymphocytes | Chemotaxis |
| ß-TG (ß-thromboglobulin) | Neutrophils | Chemotaxis; activation |
| Fibroblasts | Chemotaxis; proliferation; activation | |
| CTAP-III (connective tissue-activating peptide-III) | Neutrophils | Chemotaxis; activation |
| Fibroblasts | Chemotaxis; proliferation; activation | |
| ENA-78 (epithelial cell-derived neutrophil-activating peptide-78) | Neutrophils | Chemotaxis |
| GCP-2 (granulocyte chemotactic protein-2) | Neutrophils | Chemotaxis; activation |
| GRO-α, ß, ð (growth regulated oncogene) | Neutrophils | Chemotaxis; activation |
| Basophils | Chemotaxis; activation | |
| T-lymphocytes | Chemotaxis | |
| IL-8 (interleukin-8) | Neutrophils | Chemotaxis; activation |
| Basophils | Chemotaxis; increased of histamine release | |
| T-lymphocytes | Chemotaxis; inhibition of IL-4 synthesis | |
| B-lymphocytes | Chemotaxis; inhibition of growth and IgE production | |
| Keratinocytes | Chemotaxis; expression of HLA-DR | |
| IP-10 (interferon-inducible protein-10) | Activated T-lymphocytes | Chemotaxis |
| Monocytes | Chemotaxis | |
| NK cells | Chemotaxis; activation | |
| MIG (monokine induced by γ-interferon) | Activated T-lymphocytes | Chemotaxis |
| NK cells | Chemotaxis | |
| NAP-2 (neutrophil-activating peptide-2) | Neutrophils | Chemotaxis; activation |
| PF-4 (platelet factor-4) | Neutrophils | Chemotaxis; activation |
| Monocytes | Chemotaxis | |
| Fibroblasts | Chemotaxis | |
| Basophils | Modulation of histamine release | |
| SDF-1 (stromal cell-derived factor-1) | T-lymphocytes | Chemotaxis |
| C-C (ß) Family | ||
| Ckß8 (chemokine ß8) | Monocytes | Chemotaxis |
| Resting T-lymphocytes | Chemotaxis | |
| Eotaxin | Eosinophils | Chemotaxis |
| Basophils | Chemotaxis; activation | |
| Eotaxin-2 | Eosinophils | Chemotaxis |
| Basophils | Chemotaxis; activation | |
| Resting T-lymphocytes | Chemotaxis | |
| HCC-2 (human CC chemokine-2) | Monocytes | Chemotaxis |
| T-lymphocytes | Chemotaxis | |
| Eosinophils | Chemotaxis | |
| Monocytes | Chemotaxis | |
| MCP-1 (monocyte chemoattractant protein-1) | Monocytes | Chemotaxis |
| Basophils | Activation | |
| T-lymphocytes | Chemotaxis | |
| NK cells | Chemotaxis; activation | |
| Dendritic cells | Chemotaxis | |
| MCP-2 | Monocytes | Chemotaxis |
| T-lymphocytes | Chemotaxis | |
| Eosinophils | Chemotaxis; activation | |
| Basophils | Chemotaxis; activation | |
| NK cells | Chemotaxis; activation | |
| Dendritic cells | Chemotaxis | |
| MCP-3 | Monocytes | Chemotaxis |
| T-lymphocytes | Chemotaxis | |
| Eosinophils | Chemotaxis | |
| Basophils | Chemotaxis; activation | |
| NK cells | Chemotaxis; activation | |
| Dendritic cells | Chemotaxis | |
| MCP-4 | Eosinophils | Chemotaxis; activation |
| Monocytes | Chemotaxis | |
| T-lymphocytes | Chemotaxis | |
| Basophils | Chemotaxis; activation | |
| MIP-1α (macrophage inflammatory protein 1α or LD-78; also known as endogenous pyrogen) | T-lymphocytes | Chemotaxis |
| Monocytes/macrophages | Chemotaxis | |
| Increased IgE/IgG4 production | ||
| B-lymphocytes | Chemotaxis; activation | |
| NK cells | Activation | |
| Basophils | Chemotaxis | |
| Dendritic cells | Chemotaxis | |
| MIP-1ß | NK cells | Chemotaxis; activation |
| Dendritic cells | Chemotaxis | |
| B-lymphocytes | Increased IgE/IgG4 production | |
| NIP-3α (also known as Exodus of liver and activation-regulated chemokine [LARC]) | T-lymphocytes | Chemotaxis |
| Dendritic cells | Chemotaxis | |
| RANTES (regulated upon activation normal T-cells expressed and presumably secreted) | Eosinophils | Chemotaxis |
| T-lymphocytes | Chemotaxis | |
| Monocytes | Chemotaxis | |
| Basophils | Chemotaxis | |
| NK cells | Chemotaxis; activation | |
| B-lymphocytes | Increased IgE/IgG4 production | |
| Dendritic cells | Chemotaxis | |
| SLC (secondary lymphoid tissue chemokine) | T-lymphocytes | Chemotaxis |
| STCP-1 (stimulated T-cell chemotactic protein) | Activated T-lymphocytes | Chemotaxis |
| C (γ) Family | ||
| Lymphotactin | Lymphocytes | Chemotaxis |
| Activated NK cells | Chemotaxis; activation | |
| CX3C Family | ||
| Fractalkine | Monocytes | Chemotaxis |
| T-lymphocytes | Chemotaxis | |
Adhesion Molecules
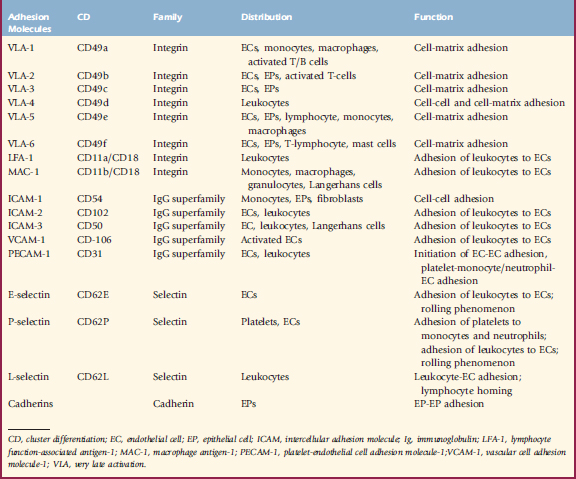
Glycoproteins critical for cell-to-cell and cell-to-matrix adhesion, contact, and communication, adhesion molecules play an integral role in cutaneous inflammation and immunology (see Chapter 1) (Table 8-4). The integrin family includes membrane glycoproteins with α and ß subunits, such as vascular cell adhesion molecule-1 (VCAM-1) on endothelial cells, which binds T-lymphocytes and monocytes via vascular leukocyte adherin-4 (VLA-4), and fibronectin and laminin, which bind keratinocytes and mast cells. The immunoglobulin gene superfamily contains intercellular adhesion molecule-1 (ICAM-1) found on keratinocytes, Langerhans cells, and endothelial cells, which bind leukocytes via leukocyte function-associated antigen-1 (LFA-1) or CD11a/CD18. The selectin family includes lectin adhesion molecule-1 (LECAM-1 or L-selectin) on lymphocytes, which binds endothelial leukocyte adhesion molecule-1 (ELAM-1 or E-selectin), and Gmp-140 (P-selectin) as a “homing” mechanism. The cadherin family is important in desmosome function (see Chapter 1).
Skin immune system
The SIS contains two major components, the cellular and humoral. The cellular component comprises keratinocytes, epidermal dendritic cells (Langerhans cells), dermal dendritic cells, lymphocytes, tissue macrophages, mast cells, endothelial cells, and granulocytes (see Chapter 1). The humoral components include Igs, complement components, fibrinolysins, cytokines, eicosanoids, neuropeptides, and antimicrobial peptides. Virtually all inflammatory and some noninflammatory skin diseases involve alterations of, or an interaction between, one or both parts of the SIS. As a result, it becomes inappropriate to consider immunologic disease as a category if one includes all skin diseases that involve the immune system. Therefore, this chapter presents those diseases classically described as allergic (hypersensitive), and Chapter 9 deals with the immune-mediated skin diseases.
Keratinocytes
Keratinocytes do much more than produce keratin, surface lipids, and intercellular substances (see Chapter 1). They are intimately associated with Langerhans cells and play a major role in the SIS. Keratinocytes produce a wide variety of cytokines that have important roles in mediating cutaneous immune responses, inflammation, wound healing, and the growth and development of certain neoplasms. Keratinocytes also produce eicosanoids, prostaglandin (PG) E2, and neuropeptides such as propiomelanocortin and α MSH. Though some of these are proinflammatory, some such as PGE2 and the neuropeptides also have anti-inflammatory effects. Keratinocytes, especially when perturbed by exposure to IFN-γ, express MHC II antigens. This expression is required for cells to be APCs for T-lymphocyte responses. Keratinocytes are capable of phagocytosis. Keratinocytes may also be stimulated to produce the leukocyte adhesion molecule, ICAM-1. They are the primary epidermal source for cytokines. Probably the most immunologically important is interleukin-1 (IL-1). Keratinocytes store IL-1, which is readily released following damage to the cells. In fact, release of IL-1 from keratinocytes is essentially a primary event in skin disease. Other cytokines derived from keratinocytes include IL-3, IL-6, IL-7, IL-8, IL-10, IL-12, IL-15, IL-16 and IL-18, TNF-α, and a variety of growth factors and granulocyte-monocyte-macrophage stimulating and activating factors. Depending on what cytokines are produced, keratinocytes may affect the type of immune response. Keratinocytes produce both IL-12 and IL-10, which may skew which type of T-lymphocytes are activated or downregulate inflammation, depending on what stage T-lymphocytes are exposed to them. Keratinocytes may also play a role in tissue repair by production of multiple growth factors. Therefore, it becomes apparent that keratinocytes are important in stimulating and controlling inflammation and repair of tissue.
Langerhans Cells
Langerhans cells are interdigitating dendritic cells, which appear as suprabasilar clear cells on skin sections stained routinely with hematoxylin and eosin (H&E) (see Chapter 1). They are members of a family of highly specialized APCs termed dendritic cells. They are localized at the interface between organism and environment and are important sentinels of the immune system. Langerhans cells are the major APCs of the epidermis. They are bone marrow-derived monocyte/macrophage-type cells. The Langerhans cell is characteristically identified in the epidermis by the electron-microscopic presence of Birbeck granules (see Chapter 1). Cutaneous dendritic APCs include both epidermal Langerhans cells and dermal dendritic cells, both of which express abundant CD1 molecules. A unique feature of CD1 antigen presentation is the ability to present nonpeptide antigens to T-lymphocytes. The epidermal Langerhans cells do not express CD90 (Thy 1), while the dermal dendritic cells do. Langerhans cells express MHC class II antigens and receptors for C3b, Fc-IgG, and Fc-IgE. The main function of Langerhans cells is antigen-specific T-lymphocyte activation. Antigenic peptides derived from endogenous protein synthesis (e.g., viral antigens, transplantation antigens, tumor-associated antigens) are generally presented in the context of MHC class I molecules, which are expressed on the surface of essentially all nucleated cells, and recognized by CD8+ antigen-specific cytotoxic T-lymphocytes. Exogenous antigens (not synthesized within APCs [e.g., extracellular bacteria, bacterial toxins, dermatophytes, vaccines, pollens, dust mites]) are presented via CD4+ helper T-lymphocytes that recognize antigenic peptides bound to MHC class II antigen selectively expressed by professional APCs (e.g., macrophages, dendritic cells, B-lymphocytes). Langerhans cells bind epidermal antigens and then present the antigens along with costimulatory molecules, the so-called “second signal” to the lymphoid tissues (regional lymph node), where helper T-lymphocytes in particular are activated. MHC II molecules are produced in the endoplasmic reticulum where they then migrate to the Golgi and endo-lysosomal compartments. The processed protein results in antigen peptides that are incorporated into the MHC II molecules at various points in this migration from endoplasmic reticulum to the cell surface. At the surface, the antigenic peptide-MHC II complex is presented to the T-cell receptor (TCR) on the surface of the T-lymphocyte, resulting in antigen-specific T-lymphocyte activation. Effective T-lymphocyte activation requires costimulators and cytokines that promote clonal expansion of the antigen-specific T-lymphocyte. The costimulator or second signal is often supplied by the expression of the B7 family of cell-surface molecules. These molecules may be expressed following Langerhans cells exposure to lipopolysaccharide, TNF-α, IL-1, and other signals. Langerhans cells produce cytokines such as IL-1 and some lipid mediators that direct the T-lymphocyte response.
Mast Cells
Mast cells are derived from hematopoietic stem cells in the bone marrow and migrate as immature unrecognizable cells in the blood and then localize in connective or mucosal tissues (see Chapter 1). Once present in tissue, they proliferate and differentiate into mature recognizable mast cells. The regulation of mast cell proliferation and differentiation is the subject of much research. Cytokines from fibroblasts, stem cell growth factor, T-lymphocytes, and IL-3 are particularly important.
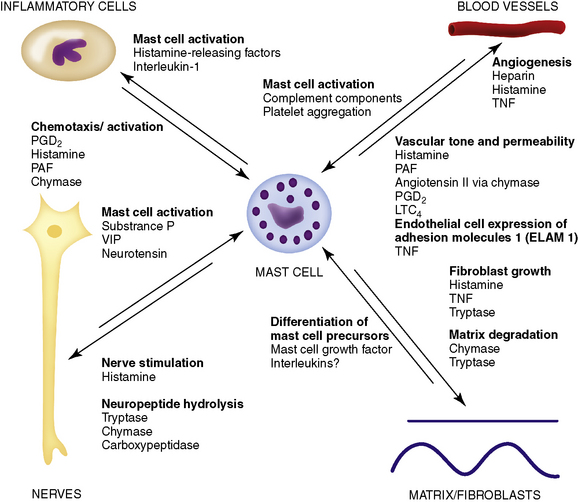
Mast cells combine characteristics of both innate and acquired immune responses: they can (1) bind certain bacteria and phagocytose/kill them, (2) elaborate and secrete several biologically active products, and (3) serve as an APC and promote clonal expansion of CD4+ helper T-lymphocytes. The importance of mast cells in immediate hypersensitivity diseases is well-documented. However, their role in other skin diseases, such as contact dermatitis and bullous pemphigoid, and in the process of fibrosis has only recently been recognized. Mast cells have diverse effects and interactions with other cells and structures of the skin (Fig. 8-1). Mast cells serve as repositories for or synthesizers of numerous inflammatory mediator substances. The mediators present vary by species studied and according to the type of the mast cells. Some mediators are universally present, such as histamine, leukotrienes, eosinophil chemotactic factor of anaphylaxis (ECF-A), and proteolytic enzymes. There are two main categories of mediators. Preformed mediators are produced and stored in mast cell granules, which are modified lysosomes that develop from the Golgi apparatus (Table 8-5). Mast cells also produce mediators that are newly synthesized at the time of activation and degranulation (Table 8-6). Mast cells have the potential to synthesize many cytokines (IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, granulocyte-macrophage colony-stimulating factor, TNF-α, IFN-γ) and many chemokines (e.g., macrophage inflammatory protein, MIP1α). Mast cells, therefore, may play many roles in the mediation of immune and inflammatory responses. Classically they are known for the recruitment of eosinophils and neutrophils, Igs, and complement from the circulation and the regulation of the immunologic response (see Tables 8-5 and 8-6). In addition, mast cells can: (1) produce IL-12 to drive Th1 responses; (2) produce IL-4, which is essential for the conversion of Th0 to Th2 cells; (3) produce IL-5 and IL-10 to drive Th2 responses; and (4) activate B-lymphocytes without surface contact.
TABLE 8-5 Preformed Mediators in Mast Cells
| Mediator | Function |
|---|---|
| Histamine | H1 and H2 receptor-mediated effects on smooth muscle, endothelial cells, and nerve endings |
| Tryptase | Cleaves C3 and C3a; degrades VIP and CGRP kallikrein-like activity; activates fibroblasts |
| Chymase | Function unclear; cleaves neuropeptides, including substance P |
| Carboxypeptidase | Acts in concert with other neutral proteases |
| Acid hydrolases | Break down complex carbohydrates |
| Arylsulfatase | Hydrolyses aromatic sulfate esters |
| Eosinophil chemotactic factor (ECF) | Eosinophil chemotaxis and “activation” |
| Neutrophil chemotactic factor (NCF) | Neutrophil chemotaxis and “activation” |
| Heparin | Anticoagulant, anticomplementary; modifies activities of other preformed mediators |
| Chondroitin sulfate | Function unknown |
| Cytokines (e.g., TNF-α, IL-4, IL-3, IL-5, and IL-6) | See Table 8-2 |
TABLE 8-6 Pharmacologic Activities of Newly Generated Mast Cell Mediators
| Mediator | Pharmacologic Actions |
|---|---|
| Prostaglandin D2 (PGD2) | Bronchoconstriction; peripheral vasodilation; coronary and pulmonary vasoconstriction; inhibition of platelet aggregation; neutrophil chemoattraction; augmentation of basophil histamine release |
| Prostaglandin F2 (PGF2) | Bronchoconstriction; peripheral vasodilation; coronary vasoconstriction; inhibition of platelet aggregation |
| Thromboxane A2 (TXA2) | Vasoconstriction; platelet aggregation; bronchoconstriction |
| Leukotriene B4 (LTB4) | Neutrophil chemotaxis, adherence and degranulation; augmentation of vascular permeability |
| Leukotriene C4 (LTC4) | Bronchoconstriction; increase in vascular permeability; arteriolar constriction |
| Leukotriene D4 (LTD4) | Bronchoconstriction; increase in vascular permeability |
| Leukotriene E4 (LTE4) | Weak bronchoconstriction; enhancement of bronchial responsiveness; increase in vascular permeability |
| Platelet-activating factor (PAF) | Platelet aggregation; chemotaxis and degranulation of eosinophils and neutrophils; increase in vascular permeability; bronchoconstriction; engenders hypotension |
Endothelial Cells
The vascular endothelium is a very active cell type that is important in inflammation, immune responses, and tissue repair (see Chapter 1). In response to various cytokines, endothelial cells express adhesion molecules (integrins, selectins, and Ig supergene family [ICAMs]) on their surfaces. The selectins (E-selectin and P-selectin) are expressed on endothelial cells following certain inflammatory stimuli and act to slow down and cause rolling of leukocytes along the vascular endothelium. The leukocyte activation results in integrin expression and binding to Ig supergene family molecules such as ICAM-1 and VCAM-1 on endothelial cells, resulting in adhesion. Transendothelial migration occurs following adhesion. Then, in response to chemokines, the migrating lymphocytes, monocytes, and granulocytes will move toward the site of inflammation. Without this ability to home, the circulating effector cells could not respond to an immunologic or inflammatory event. In addition, activated endothelial cells can synthesize and secrete numerous substances such as cytokines (including IL-1, IL-6, and IL-8), fibronectin, collagen IV, proteoglycans, blood clotting factors, growth factors, and granulocyte-macrophage colony-stimulating factor. Defects in endothelial cell adhesion molecule expression may result in disorders that mimic immunodeficiencies owing to defective migration of lymphocytes, monocytes, or granulocytes.
Granulocytes
Neutrophils have, as their major roles, the function of phagocytosis and subsequent destruction and elimination of phagocytized material. In a sense, they are the scavengers of immunologically identified debris. They are considered most important in containing infection. Owing, however, to their numerous chemoattractants (Table 8-7) and intracellular products (Table 8-8) that may be released at sites of inflammation, neutrophils are omnipresent participants in most immune and virtually all inflammatory reactions.
TABLE 8-7 Chemoattractants for Neutrophils
| Bacterial Products | Lipid Chemotactic Factors (HETE, etc.) from Mast Cells |
|---|---|
| C5a (derived from complement activation; tissue, virus, and bacterial enzymes cleave C5) | Lysosomal proteases |
| C3a | Collagen breakdown products |
| C567 | Fibrin breakdown products |
| Kallikrein | Plasminogen activator |
| Denatured protein | Prostaglandins |
| Lymphokines | Leukotrienes (especially LTB4) |
| Monokines | Immune complexes |
| Neutrophil chemotactic factor (NCF) from mast cells | |
| Eosinophil chemotactic factor (ECF) from mast cells |
| Antimicrobial Enzymes | Hydrolases |
| Lysozyme | Cathepsin B |
| Myeloperoxidase | Cathepsin D |
| N-Acetyl-ß-glucosaminidase | |
| Proteases | ß-Glucuronidase |
| Collagenase | |
| Elastase | Others |
| Cathepsin G | Lactoferrin |
| Gelatinase | Eosinophil chemotactic factor |
| Leukotrienes | |
| Pyrogen | |
| Prostaglandins | |
| Thromboxanes | |
| Platelet-activating factor |
Eosinophils, effector cells in hypersensitivity reactions, also participate in the downgrading of inflammation and defense of the host against extracellular parasites. They are also phagocytic (immune complexes, mast cell granules, aggregated Igs, and certain bacteria and fungi). Eosinophils have a tremendous ability to communicate with surrounding cells by the expression of surface receptors and cytokine secretion. Over 60 receptors for a variety of adhesion molecules, Ig Fc receptors, cytokines and lipid mediators have been found on the eosinophil membrane (Table 8-9). Eosinophil chemotaxis has been the subject of much research. Many molecules are considered good candidates for influencing eosinophil chemotaxis in vivo. These include platelet-activating factor (PAF), leukotrienes (LTB4, LTD4), dihydroxyeicosatetraenoic acid, and the C-C subfamily of chemokines. The C-C chemokines considered most potent and selective for eosinophil chemotaxis are eotaxin, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4.
TABLE 8-9 Secretory Products of Eosinophils
| Granule Proteins | Lipid Mediators |
| Major basic protein | Leukotriene B4 |
| Eosinophil peroxidase | Leukotriene C4 |
| Eosinophil cationic protein | 5-HETE |
| Eosinophil-derived neurotoxin | 5,15- and 8,15-diHETE |
| ß-Glucuronidase | 5-oxy-15-hydroxy 6,8,11,13,-HETE |
| Acid phosphatase | Prostaglandins E1 and E2 |
| Arylsulfatase | Thromboxane B2 |
| Cytokines | PAF |
| IL-1 | Enzymes |
| IL-3 | Elastase |
| IL-4 | Collagenase |
| IL-5 | Gelatinase |
| IL-6 | Reactive Oxygen Intermediates |
| IL-8 | Superoxide radical anion |
| IL-10 | H2O2 |
| IL-16 | Hydroxy Radicals |
| RANTES | |
| TNF-α | |
| TGF-ß | |
| MIP-1α |
ETE, eicosatetraenoic acid; diHETE, dihydroxyeicosatetraenoic acid; HETE, hydrocyeicosatetraenoic acid; IL, interleukin; MIP, macrophage inflammatory protein; PAF, platelet-activating factor; TGF, transforming growth factor; TNF, tumor necrosis factor.
Humoral Components
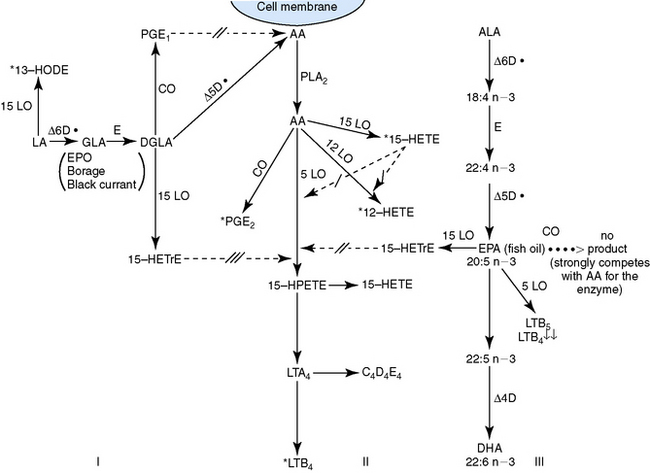
Leukotrienes (LTA, LTB, LTC, LTD and LTE) and their precursors—hydroperoxyeicosatetraenoic acids (HPETEs) and hydroxyeicosatetraenoic acids (HETEs)—are derived by the metabolism of arachidonic acid by the three enzymes of lipoxygenation, 5-, 12-, and 15-lipoxygenase. Different tissues express variable levels of the cytosolic LOXs. The 5-lipoxygenase pathway predominates in neutrophils, monocytes, macrophages, and mast cells, whereas the 15-lipoxygenase pathway predominates in eosinophils and in endothelial and epithelial cells. The 12-lipoxygenase pathway predominates in platelets. The 5-lipoxygenase pathway results in the production of LTA4 that, depending on the enzymes present in the cells, is converted to LTB4, or LTC4, and LTD4 or LTE4 are produced from LTC4. Typically, eicosanoids have autocrine and paracrine functions that are important locally for host defense, and then they are inactivated or degraded. Abnormalities in production or control mechanisms may occur, however, leading to local or systemic tissue damage and disease. The actions of eicosanoids are quite diverse and variable according to the species, tissue, cellular source, the presence of stereospecific receptors, and the generation of secondary mediators. The effects of arachidonic acid formation and some of the activities that eicosanoids may have in skin disease are summarized in Table 8-10.
TABLE 8-10 Effects of Eicosanoids in Skin Disease
| Eicosanoid | Effect |
|---|---|
| LTC1/D1/E1 | Vascular dilation and increased permeability |
| LTB4 | Leukocyte chemotaxis and activation; increased endothelial adherence of leukocytes; stimulates keratinocyte proliferation; enhances NK cell activity; hyperalgesia |
| 12-HETE | Stimulates smooth muscle contraction |
| 15-HETE | Hyperalgesia; inhibits cyclooxygenase; inhibits mixed lymphocyte reaction; stimulates suppressor T-cells; inhibits NK cell activity |
| 15-HPETE | Suppresses T-lymphocyte function and Fc receptors |
| PGE2 | Plasma exudation; hyperalgesia; stimulates cell proliferation; suppresses lymphocyte and neutrophil function |
| PGF2 | Vasoconstriction; synergy with histamine and bradykinin on vascular permeability; stimulates cell proliferation |
| PGD2 | Smooth muscle relaxation |
| PGD2/PGI2 | Suppression of leukocyte function; vasodilation and increased permeability |
Types of hypersensitivity reactions
Clinical hypersensitivity disorders were divided on an immunopathologic basis, by Gell and Coombs, into four types:5,8
Therapy for hypersensitivity disorders
Treatment Plans
Most horses with chronic allergies, particularly those with atopic dermatitis, require a combination of therapeutic agents for optimal control of symptoms.* When possible, the optimum treatment of all allergies is avoidance of the offending allergen(s). If this is not possible, most treatments are directed at blocking the effects of the allergic reaction. Prevention may also occur with allergen-specific immunotherapy (ASIT) (hyposensitization, desensitization) (see discussion on Atopic Dermatitis) and, possibly, with some immunomodulatory drugs. When allergen avoidance and/or ASIT are ineffective, partially effective, or when clients decline these approaches, other medical options will be required. Often the optimum control will require different therapeutic protocols over the life of the animal. Therefore, a treatment plan will be required that takes the different problems, goals of the clients, and types of therapeutic agents into consideration. In general, these are aimed at treating the by-products of the allergic reaction that has already occurred. The majority of therapeutic protocols are used to help alleviate pruritus, the major symptom of most allergic diseases, to treat specific problems or secondary infections, to avoid or decrease exposure to offending allergens, and to decrease inflammation. These therapeutic protocols may be specific for a problem or etiology or nonspecific. The most commonly recommended therapeutic protocols are listed in Table 8-11. These treatment options vary in their ease of administration, risks, efficacy, expense, and monitoring required. In many cases, combinations of treatments may be used, and an overall plan to control those aspects of the problem considered most bothersome by the client is the most effective way to manage these cases long term.
TABLE 8-11 Therapeutic Regimens
| Nonspecific Therapy | Specific Therapy |
|---|---|
| Soothing topical baths, rinses | Antibiotics |
| Moisturizers | Insect control |
| Topical glucocorticoids | Allergen-specific immunotherapy (hyposensitization) |
| Fatty acids | Novel protein (“hypoallergenic”) diet |
| Nonsteroidal anti-inflammatory agents (e.g., pentoxifylline) | Immunosuppressive therapy |
| Antihistamines | |
| Antidepressants | |
| Systemic glucocorticoids |
Topical Therapy
Topical therapy is often incorporated into treatment plans and is discussed in more detail in Chapter 3.7 The major disadvantages are the time and effort needed for administration. Expense may also be an important factor. Total body bathing and/or rinses are required for regional or generalized pruritus and often reduce pruritus by rehydrating the stratum corneum and by removing surface debris, microbial by-products, and allergens that may contribute to the pruritic load. Hydrocortisone (1%) shampoo is not significantly absorbed and may help treat allergic reactions. Treatment with ointments, creams, lotions, and sprays may be possible for localized areas. Topical glucocorticoids are most effective, but generally limited to localized allergic reactions. Cold water, hypoallergenic and moisturizing shampoos and creme rinses, colloidal oatmeal, and shampoos and creme rinses containing pramoxine (local anesthetic) can reduce pruritus for up to 72 h (see Chapter 3). Topical therapy unfortunately is often overlooked and not presented as an option to clients. Appropriately administered topical therapy can reduce the need for systemic treatment in many patients.
Systemic Therapy
Several systemic agents can be used in the medical management of allergic horses. The clinician needs to be familiar with which of these agents must be withdrawn prior to shows and competitions. Medicines capable of improving racing performance of horses are classified by the Association of Racing Commissioners International (ARCI) based on their performance-enhancing potential (www.arci.com). Antihistamines are in Classes 3 and 4, and glucocorticoids are in Class 3.
Fatty Acids
Fatty acids and their role in normal skin and coat are discussed in Chapter 3. As these agents are relatively benign, diets or supplements containing appropriate amounts and ratios could be used in most allergic horses.
EPA, which is usually supplied by using cold water marine fish oils, also competes as a substrate for COX and 5- and 15-lipoxygenase. The metabolism of EPA by the LOX enzymes results in the formation of LTB5 and 15-hydroxyeicosapentaenoic acid. These two products are believed to inhibit LTB4, which is a potent proinflammatory mediator. Fig. 8-2 demonstrates the interactions of GLA, EPA, and arachidonic acid. Flaxseed contains α-linolenic acid, which is metabolized to EPA.
Little information has been published on the usefulness of these fatty acids in horses with inflammatory dermatoses. The oral administration of linseed or flaxseed oil (50-58% α-linolenic, 13-18% linoleic) inhibited inflammation, the production of eicosanoids, and the production of TNF in experimental studies in horses.7,11,12 Although linseed oil and flaxseed oil are both produced from flaxseed, the extraction processes are different. Linseed oil is extracted under conditions of high temperatures and petroleum extraction. Flaxseed oil is cold pressed with no solvent used. Linseed oil may cause depression, anorexia, and mild colic in horses, whereas flaxseed oil does not.7 In two double-blinded, placebo-controlled clinical studies, no significant reduction in pruritus occurred in allergic horses treated orally (PO) with linseed oil or a commercial product (evening primrose oil and cold water marine fish oil).7 These studies are difficult to interpret because: (1) it was not clear how many horses had insect-bite hypersensitivity, atopic dermatitis, or both; and (2) the horses’ base diets were not analyzed. In addition, as horses may have low levels of Δ-6-desaturase and Δ-5-desaturase activity,12 supplementation with specific omega-3 (e.g., EPA) and omega-6 (e.g., GLA) fatty acids may be more effective. A milled flaxseed supplement reduced reactivity to intradermal injections of Culicoides antigen in horses with insect-bite hypersensitivity.19
Many clinicians feel that omega-6/omega-3 fatty acid supplementation is beneficial in some allergic horses, especially those with atopic dermatitis.* Although fish oils are occasionally unpalatable to some horses, a commercial fatty acid supplement (DVM Derm Caps 100s), given PO at 1 capsule/50-100 kg every 12 h appears to be well-tolerated and effective in some horses. Platinum Performance Equine Wellness and Performance Formula (omega-3 fatty acids, tract minerals, vitamins, antioxidants) and Platinum Vet Skin and Allergy Formula (algal omega-3 fatty acids, quercetin, purified calf thymus) are popular commercial products, but no scientific studies substantiate their benefits in equine skin disease. These fatty acid supplements may also have synergistic effects when administered in conjunction with glucocorticoids and/or antihistamines.7,23,46 Fatty acid supplements should be given for 3-12 weeks before a judgment is made as to their benefits.
Nonsteroidal Anti-inflammatory Agents
Nonsteroidal anti-inflammatory agents are classically used to decrease pain and inflammation, but have shown little benefit for pruritus or atopic disease. A number of nonsteroidal drugs have been used to treat equine pruritus. Little or no work has been done to document the effect of these, including phenylbutazone, diethylcarbamazine, flunixin, ketoprofen, orgotein (metalloprotein, nonsteroidal anti-inflammatory), phenothiazine tranquilizers, barbiturates, and levamisole.7 Experimental studies have indicated that 5-lipoxygenase inhibitors, a leukotriene synthesis inhibitor, and PAF receptor antagonists inhibit various aspects of the equine inflammatory response,7 but therapeutic trials with such agents have not been reported. Montelukast (a leukotriene receptor antagonist) administered PO at 0.11 mg/kg/day was of no benefit in horses with chronic obstructive pulmonary disease (COPD).13
Pentoxifylline has a variety of immunomodulatory effects, and the benefits may result from different mechanisms, depending on the disease being treated (see Chapter 9).14,15,21 Pentoxifylline may be useful—especially for reducing required glucocorticoid doses and frequencies—in some allergic horses.7,23
Methylsulfonylmethane (MSM EQ, Vetri-Science) is an organosulfur compound and a metabolite of dimethyl sulfoxide. It is recommended by the manufacturer to “support proper joint function and connective tissue health” in horses. Anecdotal reports indicate that MSM can be used in combination with other antipruritic agents in allergic horses.6,23 The powder is sprinkled on the food at 10-12 gm/500 kg every 12 h, and then every 24 h. The product is well-tolerated, but its benefits in equine dermatology are unproven.
Antihistamines
H1 receptors are primarily responsible for pruritus, increased vascular permeability, release of inflammatory mediators, and recruitment of inflammatory cells. In addition to their histamine-blocking action, some of these antihistamines have sedative, antinausea, anticholinergic, antiserotoninergic, and local anesthetic effects. They will block mediator release if present prior to allergen challenge and if at the appropriate concentration. Most of the second-generation, or nonsedating, antihistamines block mediator release. Some, such as cetirizine, also block the allergen-induced late-phase cutaneous reaction, decrease the influx of eosinophils, and downregulate TNF-α-induced hyperactivation of nuclear factor kappa beta (NF-κβ).17,18 Second-generation antihistamines (less likely to cross blood-brain barrier) typically have less side effects than first-generation antihistamines (cross blood-brain barrier).
There is little published information on the use of antihistamines in horses. Most clinicians consider hydroxyzine (1-2 mg/kg every 8-12 h PO) to be the antihistamine of choice in the horse (Table 8-12).* It is not known what percentage of horses respond to any given antihistamine. However, as the effects of different antihistamines vary from one horse to another, it is important to try several different ones before concluding that the pruritus or inflammation is not responsive to these agents. Other antihistamines suggested for use in allergic horses include chlorpheniramine (0.25-0.5 mg/kg every 12 h PO) and diphenhydramine (1-2 mg/kg every 8-12 h PO). Tripelenamine and pyrilamine are not usually effective.7,10,23 Clemastine and fexofenadine have very poor oral bioavailability in horses and are unlikely to be clinically useful.7,16,22 Cetirizine (0.2-0.4 mg/kg every 12 h PO) has good bioavailability in horses and could be clinically useful,17,18 but no clinical trials have been reported. Antihistamines should be given for at least 2 weeks before a judgment is made as to their usefulness. Antihistamines may act synergistically with glucocorticoids and/or fatty acids in pruritic horses. Antihistamine side effects are rare in the horse and include sedation, lethargy, and behavioral changes.
TABLE 8-12 Antihistamines and Antidespressants Used in Allergic Horses*
| Antihistamine | Dose | Frequency |
|---|---|---|
| Amitriptyline | 1 mg/kg | Every 12 h |
| Chlorpheniramine | 0.25-0.5 mg/kg | Every 12 h |
| Diphenhydramine | 1-2 mg/kg |