The remaining 1% body weight is transcellular fluid, this is specialised excreted fluids that serve a specific function, e.g. cerebrospinal fluid, gastrointestinal tract secretions.
Intravascular volume comprises such a small proportion of total body fluid that any loss of fluid from this compartment (hypovolaemia) has much more severe physiological effects than global loss of fluid (dehydration).
The body water content will depend on the balance between the amount of water that is acquired by the body and the amount that is lost.
Normal water intake:
- Drinking
- Eating: moist diets may be 70–80% water
- Metabolism: oxidation of fat, carbohydrate, protein produces water
- Therapeutic.
Normal fluid loss:
- Urination: regulated by healthy kidneys, fluid and electrolytes
- Defecation: small amounts, fluid and electrolytes
- Respiration: evaporation, water only
- Sweating: small amount in cats and dogs, fluid and electrolytes.
In some situation, or illnesses, the animal’s ability to maintain the body’s fluid balance will be impaired.
Abnormal water intake:
- Metabolic disorders
- Anaesthesia (pre-operative/general anaesthetia/recovery)
- Systemic illness
- Dysphagia, physical difficulty (see Figure 4.2)
- Water deprivation.
Figure 4.2 A patient with a fractured mandible following a road traffic accident. Physical difficulty in drinking may reduce water intake.

Abnormal fluid loss:
- Vomiting (4 ml/kg per vomit.)
- Diarrhoea (4 ml/kg per episode, up to 200 ml/kg/day)
- Abnormal urine production: renal disease, diabetes
- Increased respiratory evaporation: e.g. panting, dyspnoea
- Pathological fluid losses: transudate, exudates, etc. Pyometra, burns, peritonitis
- Haemorrhage
- Surgery: evaporation from surgical site, haemorrhage, etc.
Abnormal Losses from Fluid Compartments
When considering administering fluid therapy to correct a fluid deficit, it is essential to think carefully about where the deficit exists. This should be straightforward based on the history of the patient, and also a clinical examination.
The history of the animal will give an idea of the type of fluid lost:
Dehydration and hypovolaemia are not interchangeable terms. Dehydration refers to a hydration deficit, where water is lost over the whole of the body, but predominantly from the intracellular and interstitial fluids. Hypovolaemia refers to a reduction of intravascular volume which therefore reduces the perfusion of tissues, causing a perfusion deficit.
Both hydration and perfusion parameters are initially assessed with physical examination. Hydration status is assessed by looking at parameters such as moisture of mucous membranes, skin turgor and presence of retraction of the globe, which are affected by interstitial and intracellular fluid levels. Perfusion status is assessed by physical parameters that are affected by intravascular volume and perfusion: heart rate, pulse quality, mucous membrane colour, capillary refill time, urine output.
The two conditions cause distinctly different clinical signs and must be managed in different ways, with different fluids and at different rates of administration, so appreciating the difference, and recognising the symptoms, is essential in formulating a treatment plan.
Forming a Fluid Therapy Plan
When formulating a fluid therapy plan for a patient, it is worth considering the following questions:
- Does the patient need fluid therapy?
- Which route should be used?
- Which fluid should be administered?
- At what rate, for how long?
Does the Patient Need Fluid Therapy?
The history of the presenting animal will be a good indicator in determining where the deficit exists: whether a hydration deficit or a perfusion deficit. The history should include duration of illness, frequency of vomiting or diarrhoea, water intake, food intake and any haemorrhage, to get an idea of fluid losses and intakes.
Perfusion Deficits: Hypoperfusion
The term ‘shock’ is used where oxygen delivery to tissues is poor due to tissue hypoperfusion. This leads to cell damage, and if not corrected to organ dysfunction, organ failure and death. Cell damage and cell death will lead to the release of inflammatory mediators which can lead to systemic inflammatory response syndrome (SIRS).
Tissues may be hypoperfused due to:
- Decreased circulating blood volume (hypovolaemic shock)
- Decreased capacity of blood to deliver oxygen
- Decreased ability of the heart to pump blood (cardiogenic shock)
- Decreased ability of vascular system to maintain vasomotor tone (maldistributive shock)
- Obstruction of blood flow from, or to, the heart (obstructive shock).
Hypovolaemic Shock
Hypovolaemic shock is the most common form of shock seen in veterinary medicine, where tissue hypoperfusion is due to loss of circulating blood volume. This loss of volume may be due to blood loss (internal or external), or loss of fluid from the gastrointestinal tract (vomiting, diarrhoea), the kidneys, or effusion and/or transudate into the peritoneal or pleural space. (Strictly speaking the term dehydration refers to loss of water from the whole of the body – a global loss. If dehydration is severe enough it may lead to hypovolaemia, but the two terms are not the same – hypovolaemia refers to loss of intravascular fluid, and does not include extravascular fluid (intracellular and interstitial).)
If the circulating blood volume falls, the body’s defence mechanisms come into play. Blood flow is diverted away from capillary beds that are less essential (e.g. skin, gastrointestinal tract) so that the circulating volume can be sent to more ‘vital’ organs such as the brain, heart and kidneys. This is achieved by vasoconstriction of the vessels leading to the capillaries of the less vital organs. This is a normal solution to a problem, and saves lives, but volume needs to be restored before irreversible cell damage occurs.
Recognising Hypovolaemic Shock
A diagnosis of hypovolaemic shock is made by a careful physical examination, whereby signs of poor tissue perfusion can be recognised:
The extent of the alteration in perfusion parameter should give the clinician an accurate idea of the severity of hypovolaemic shock present (see Table 4.1).
Table 4.1 Detecting changes in perfusion parameters

Physical examination is the main basis on which hypovolaemic shock is recognised, but laboratory samples can be of some use. Lactate is produced when cells are metabolising anaerobically, i.e. without oxygen. If tissues are poorly perfused, oxygen delivery is reduced and lactate production increases. Due to poor perfusion, lactate clearance is also decreased (see Chapter 7) so increased lactate levels in blood are an indicator of poor perfusion, and in some cases can be used as a prognostic indicator.
Maldistributive Shock
In maldistributive shock, poor perfusion of tissues exists due to loss of vasomotor tone and therefore inappropriate vasodilation. This leads to the intravascular fluid being distributed across the body in an abnormal way, the body has effectively lost control of the regulation of perfusion. Clinical signs of maldistributive shock include rapid capillary refill time (CRT), red mucous membranes and tachycardia.
Causes of maldistributive shock are anaphylaxis or SIRS (see Figure 4.4). SIRS is a clinical state where a localised pathology has lead to widespread systemic inflammation, associated with dilation and increased permeability of blood vessels. Initiating causes may be infectious (sepsis) or non-infectious (pyometra, septic peritonitis, pancreatitis, severe tissue injury, burns, neoplasia).
Figure 4.4 A patient with systemic inflammatory response syndrome (SIRS). The brick-red mucous membranes are caused by vasodilation due to loss of vasomotor tone.

Hydration Deficits
Hydration status is classically assessed by measuring physical parameters that will be affected by reduction of fluid in the interstitial and extracellular fluid. Assessment includes:
- Moistness of gums or cornea: other problems such as nausea can cause excessive salivation and make membranes appear moist even if dehydrated
- Skin turgor or skin tenting: dehydration causes the skin to remain tented for several seconds
- Retraction of the globe (sunken eyes).
This assessment can only give a very rough approximation of deficit at best (see Table 4.2). Signs of dehydration can be very variable; skin turgor can vary as the animal gets older and with body condition (see Figure 4.5). The assessment may often underestimate the degree of dehydration, but this is not always a problem as, in contrast to hypoperfusion, these losses need to be replaced more gradually. Any animal that is showing any signs of hypoperfusion should be treated immediately and hydration status checked later once hypoperfusion is corrected.
Table 4.2 Physical indicators of hydration deficits
| Percentage dehydration | Clinical signs |
| <5% | No detectable clinical signs Increased urine concentration |
| 5–6% | Subtle loss of skin elasticity (tenting) |
| 6–8% | Marked loss skin elasticity Slightly sunken eyes Dry mucous membranes |
| 10–12% | Tented skin stays in place Sunken eyes, protruded third eyelid Dry mucous membranes Progressive signs of shock |
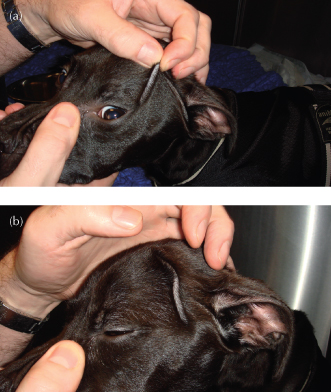
Figure 4.5 Skin tenting can be affected by body condition: (a) performing a skin tenting test in an emaciated dog; (b) there is a persistent skin tent long after releasing the skin due to absence of subcutaneous fat.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



