Chapter 71 Rhinoceros Theriogenology
Over the last few decades, the six rhinoceros species have become important icons in the saga of wildlife conservation.12 Recent surveys have estimated the wild black (Diceros bicornis), southern white (Ceratotherium simum), northern white (Cerathoterium cottoni), Indian (Rhinoceros unicornis), Javan (Rhinoceros sondaicus), and Sumatran rhinoceros (Dicerorhinus Sumatrensis) populations to be, at most, 3610, 11,330, 7, 2500, 70, and 300, respectively. Protected against habitat loss, poaching, and left undisturbed, rhinoceroses reproduce well in the wild. However, small and decreasing populations make successful captive management increasingly important.
From the first descriptions of the reproductive anatomy and estrous cycle to the present use of advanced assisted reproduction technologies, researchers and veterinarians have attempted to understand the function and dysfunction of the reproductive biology of these charismatic species.10,22 This chapter briefly reviews current knowledge on rhinoceros theriogenology.
Male Reproductive Anatomy and Clinical Aspects
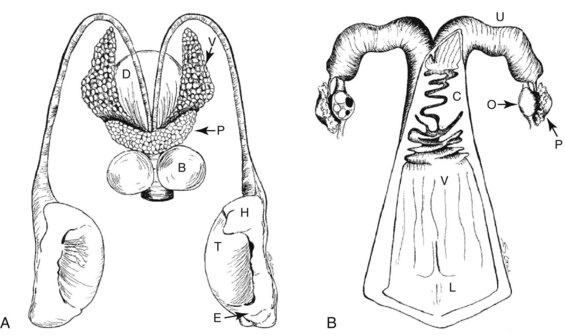
The external and internal genitalia of the male rhinoceros include the prepuce, prepucial fold, penis, accessory sex glands, duct deferens, epididymis, and testes (Fig. 71-1).44–4759
The relaxed musculocavernous penis is fully covered by the prepucial fold. During urination and territorial marking, the penis exits the sheath, pointing caudally between the hind legs. The fully erect penis points cranially; it has characteristic horizontal flaps and a mushroom-shaped process glandis, both being specific to each species. The horizontal flaps and process glandis suggest that the rhinoceros is a cervical inseminator. During up to a 45- to 60-minute intromission, the flaps unfold in the female’s vagina while the process glandis locks into the portio and cervical folds.44,59
The paired set of accessory sex glands consists of cigar-shaped, multilobulated, seminal glands, the prostate with two triangular lobes and an isthmus across the urethra, and compact, round, or elongated bulbourethral glands. An ampulla at the end of the deferens ductus has not been described for the rhinoceros. In adult white rhinoceros, it has been shown that the volume of the accessory sex glands correlates with semen quality.45,47,59
The testes and tightly attached epididymides are located in the dorsal aspect of the prepucial fold. Their position in the prepucial fold shifts from vertical, clearly visible and palpable, caudal to the relaxed penis to more horizontal and nonpalpable, adjacent to the inguinal rings.45,47,59 Thick skin, dense testicular capsule and changing positions limit possible conclusions from testicular palpation about functional status or pathologic lesions. Clinical examination of the testes and accessory sex glands relies on transcutaneous and transrectal ultrasound, respectively. Whereas transrectal examination of the accessory sex glands requires a chute-trained animal or sedation, transcutaneous ultrasound of the testes can be achieved through the bars of the enclosure with very little training.
Semen Collection, Evaluation, and Preservation
The evaluation of breeding soundness to determine a bull’s reproductive potential consists of two components, clinical examination of the reproductive tract and semen collection. Semen collection and evaluation in rhinoceros are aimed at the determination of causes for reproductive failure or for semen donation and preservation for assisted reproduction for males with known, good semen quality. Values generated might therefore differ greatly and their interpretation must be put carefully in context with the method of collection, the male’s libido, presence or absence of mating or masturbation, territorial behavior, and diagnosed reproductive tract disease. Semen quality provides indications on the current breeding potential of a male. Poor semen quality does not exclude remaining chances of fertilization but is mostly associated with a lack of breeding activity.17,36,41,44,53
Semen Collection
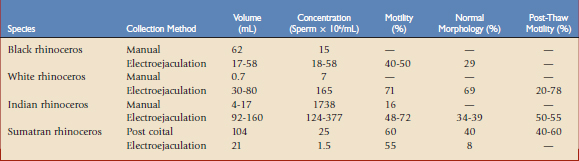
Semen can be collected postcoitally, by manual stimulation, rectal massage, or electroejaculation, or postmortem (Table 71-1).14,38 Postcoital collection, manual stimulation, and rectal massage give regular access to sperm for preservation and cryopreservation and later use in assisted reproduction without medical restraint. During postcoital semen collection, the ejaculatory fluid is captured draining from the vulva. Mating behavior, proper intromission, and ejaculation are prerequisites for this collection method, but are usually absent in males for which semen evaluation is warranted. Manual stimulation with or without light sedation has been successful in individual black, white, and Indian rhinoceroses. However, it is time-consuming, limited to well-trained individuals, and requires a restraint chute. It is characterized by inconsistent collection success and sometimes only a minute ejaculate volume 0.2 mL. Attempts to collect semen by the use of an artificial vagina have produced ejaculates but failed to produce sperm.
Electroejaculation under general anaesthesia is the method of choice for obtaining a semen sample in untrained, captive, or wild rhinoceroses. It has been adapted and applied in all captive rhinoceros species and provides reliable sampling for semen evaluation, assessment of current breeding potential, and sperm preservation for assisted reproduction purposes.* Prior to electrostimulation, ultrasound helps determine the position of the accessory sex glands, facilitating accurate placement of the electrostimulation probe and avoiding urine contamination from accidental stimulation of the bladder or bladder neck. The electrostimulation probe is placed onto the pelvic part of the urethra and prostate. Electric current induces ejaculatory contraction of the accessory sex glands and pelvic urethra. Manual massage of the pelvic and penile urethra between stimulation sets funnels the emitted ejaculatory fluids into the thermally and light-insulated collection tube. Sperm-rich fractions are emitted at the beginning of the induced ejaculatory process, followed by less concentrated fractions of sperm and aspermatic seminal plasma.
The core of semen evaluation is based on the sperm-rich fractions of the ejaculate. Semen evaluation involves assessment of ejaculate volume, color and odor, and sperm motility, morphology, viability and concentration (see Table 71-1). In general, ejaculates can be obtained from bulls as young as 6 and up to 42 years of age. Sperm quality is not affected by age and can remain high until advanced age, despite the presence of increasing testicular fibrosis. Sperm production and androgen concentration are consistent throughout the year and do not show seasonal influences.17 Recorded ejaculate volumes range from 0.1 to 200 mL and seem to be greatly influenced by collection method, probe operator, probe design, or stimulation protocol applied. Ejaculates collected postcoitally or by electroejaculation show the most consistent mean volumes, from 17 to 160 mL, presumably representing the naturally ejaculated volume range.The preparation of epidydimal sperm after castration or postmortem represents a unique opportunity for a final male gamete rescue. The extraction of spermatozoa from the epidymis or vas deferens rescues the remaining stock of mature gametes. Extraction is best performed on site shortly after the donor’s death to avoid autolysis or heterolysis of the tissue and temperature changes affecting sperm quality. Cooled transport (4° C) of the testis and epidymis in saline solution is an alternative to on-site preparation if the death occurs unexpected Cryopreserved sperm conserves the male’s ability to produce offspring posthumously. The authors used sperm extracted and cryopreserved postmortem for artificial inseminations, which have resulted in pregnancy.
Semen Assessment and Preservation
Rhinoceros spermatozoa are similar in size to those of the stallion.2 Sperm characteristics show huge ranges (see Table 71-1). Good- and poor-quality semen has no significant differences in terms of ejaculate volume, pH (8.5 to 9.0), or osmolarity (290 to 300 mOsm/kg).53 Sperm motility, viability, and morphology therefore represent the most relevant indicators to rate current semen quality. In domestic species, sperm motility and morphology are positively correlated with pregnancy rate. In the rhinoceros, analogous to domestic species, the percentages of motile and morphologically normal sperm in sperm-rich fractions are used to rate a bull’s semen quality. Semen quality is considered as a prognostic indicator of the sperm’s fertilizing potential and thus of the bull’s breeding potential. Sperm with motility of 75% is considered to have excellent fertilizing potential. Fertilizing potential of sperm with motility of less than 75% or less than 50% is considered intermediate or poor.17,36 However, samples with 60% motility have proven sufficient for assisted reproduction and semen preservation.25 Variations in semen quality among collections may occur and repeated evaluations are suggested to confirm poor semen quality and limited breeding potential of a bull. In white rhinoceroses, social structure and subordinate behavior have been identified as a cause of the high incidence of reduced semen quality in males without or long past breeding history. The subordination of males in multimale institutions has corresponded with low sperm quality. Socially subordinated males display decreased reproductive fitness in the presence of a territorial male. Altering the captive social structure by separating nonbreeding but territorial males, exclusive access of subordinate males to breeding females, or introduction of new challenging males might improve libido and reproductive parameters.
Rhinoceros spermatozoa are not sensitive to slow chilling. The motility of white rhinoceros sperm chilled to 4° C after suspension in equine, bovine, or custom-made extenders remains almost constant for 24 hours.17 In another study, we noted that high-quality sperm from Indian and black white rhinoceroses show reduced motility values after only 72 hours of chilling. This low sensitivity of rhinoceros sperm to chilling facilitates sperm transport to distant locations for use in regional AI breeding programs.20
Cryopreservation of sperm has been shown in white, Sumatran, and Indian rhinoceroses and was successfully used for artificial insemination in white and Indian rhinoceroses.* Cryopreservation of male gametes permits the use of sperm from unrepresented, wild, or deceased males, thus improving the genetic diversity of a population. In general, cryopreservation of sperm induces cell damage, resulting in cell death or loss of function. Motility, viability, morphology, and acrosome integrity of the sperm are the main characteristics used to assess semen quality after thawing. Single- or double-stranded sperm DNA fragmentation has recently been added as a critical parameter for assessing post-thaw sperm integrity.32 Fast fragmentation of the rhinoceros sperm nucleus in comparison with other eutherian species, including the stallion, suggests that semen processing and current freezing methods used will need further substantial improvement to increase cell and DNA integrity and longevity of rhinoceros sperm after cryopreservation.
Cryopreservation of Sperm
A critical precondition to cryopreserving rhinoceros sperm is a high-quality semen sample. Even in one ejaculate, only few sperm-rich fractions with highly motile sperm qualify for preservation. Standard or modified equine extenders or a skim milk–egg yolk or TEST–egg yolk medium are used to cryopreserve the sperm, with good success. Glycerol and dimethylsulfoxide (Me2SO) in concentrations of 5% or 6.25% are used as cryoprotectants.17,24,25 In a Sumatran rhinoceros, Me2SO was identified as the superior cryoprotectant compared with glycerol. In large-scale studies in white rhinoceroses, Me2SO, as an exclusively tested cryoprotectant, produced reproducible good results.36 However, in a recent comparative study in Indian rhinoceros, no significant difference in post-thaw results was found between glycerol and Me2SO.53 Sperm is generally frozen in 0.5-mL straws in liquid nitrogen vapor. This freezing method provides good post-thaw results across species, and is relatively simple and easy to apply under field conditions. A new freezing technology, multithermal gradient directional freezing (Core Dynamics, Orangeburg, NY), facilitates freezing of large volumes (8 mL/vial).16 Thanks to precise control over ice crystal formation, it is reported to result in less cell damage and higher gamete survival. In a comparative study, directional freezing has been superior to freezing over liquid nitrogen vapor in white rhinoceroses. However, high liquid nitrogen consumption and vulnerable electronics make this technology logistically more challenging.
Diseases of the Male Reproductive Tract
Penis
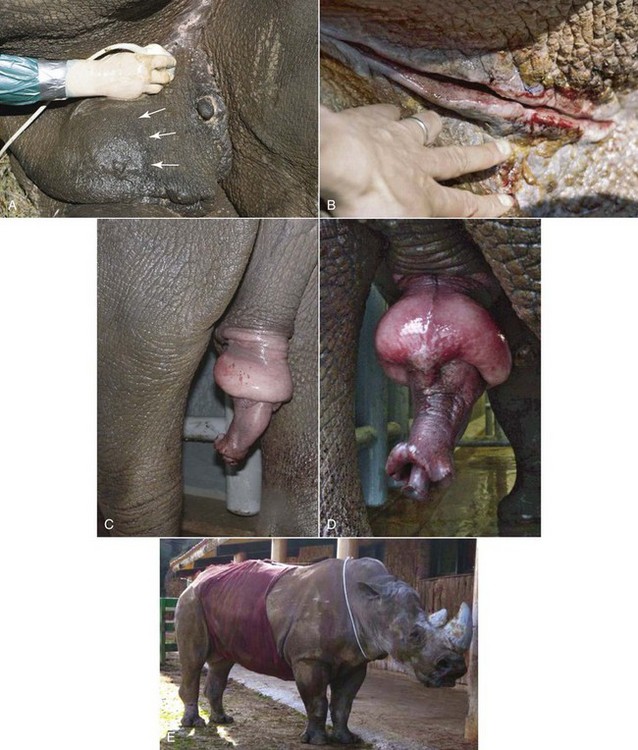
Mating or masturbation on foreign objects may cause trauma to the penis, resulting in superficial lesions, edema, or abrasion (Fig. 71-2). Superficial lesions are treated by and responsive to topical ointment. More severe abrasions might cause edema further, preventing retraction of the penis. With the penis permanently unprotected, edema increase and further secondary abrasions might occur to the free-hanging and swinging penis. Similar to the stallion, blood circulation of the glans is disturbed and aggressive therapy is necessary to prevent further abrasions, ulceration, and irreversible penile damage. Replacement of the penis into the sheath is the primary goal, best achieved by bandaging the penis to the abdominal wall. Blunt trauma of the erect penis can cause penile fracture, resulting in inability to obtain intromission thereafter.21
Testis
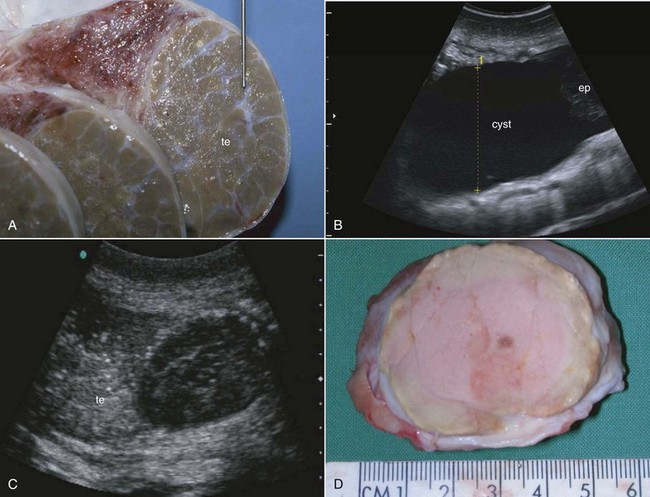
Testicular fibrosis, atrophy, trauma, or neoplasia and epididymidal cysts are disorders described in the male rhinoceros (Fig. 71-3).17,30,31,53 Testicular fibrosis is a common finding in older males. In white and Indian rhinoceroses, testicular fibrosis starts forming at the age of 15 years.17,53 Fibrotic areas, located in the interstitium, appear in the ultrasound as bright dots (approximately 0.1-0.3 mm) within the testicular parenchyma. The size and number of fibrotic spots are positively correlated with age and are regarded as signs of testicular aging, but with no influence on semen characteristics.
Different from age related fibrosis, physical trauma, testicular neoplasia or epididydimal cysts may influence sperm production, causing subfertility or infertility.30,31 During territorial fights or rough courtship, the opponent’s horn forks into the inguinal area of the male, causing blunt trauma of the gonads (see Fig. 71-2). Hematoma or seroma of the testis may be visible as asymmetric swelling of the prepucial fold or by visualizing fluid cavities in and around the testis using ultrasound. Effects of hematoma or seroma on sperm production might be temporary. However, severe trauma might induce testicular necrosis and atrophy or permanent stenosis of the spermatic cord, resulting in infertility.
Testicular neoplasia is characterized as solid mass within the testicular parenchyma (see Fig. 71-3).30,31 Diagnosed by ultrasound, it is further characterized by testicular biopsy. Seminoma has been described in black and white rhinoceroses. Hemicastration of the affected testis prevents further growth and possible metastasis and ensures the animal’s breeding potential. A hemicastrated black rhinoceros resumed good-quality semen production after surgery, with proven fertility.
Epidydimal cysts as a defect of the gonaduct system have been detected in Sumatran and white rhinoceroses by the authors and Schaffer43 (see Fig. 71-3). Filled with clear fluid, their dimensions can range from 1 to 10 cm. Ejaculates from effected males are consistently aspermatic or of low quality. The cause of these cysts is unclear. Previous trauma resulting in the formation of cysts might induce seroma and stenosis of the gonaduct system. Transcutaneous fine-needle aspiration of the cystic fluid can be attempted to resolve the cyst(s), resuming sperm passage and fertility.
Female Reproductive Anatomy and Clinical Aspects
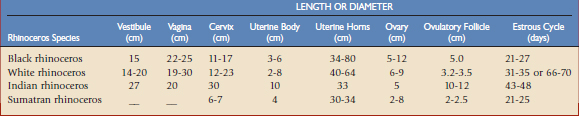
The female reproductive organs in the rhinoceros consist of the vulva, clitoris, vestibule, vagina, cervix, uterus bicornis, oviduct, and ovaries. The total length of the genital tract varies among species, from approximately 50 cm in the Sumatran to well over 100 cm in the white rhinoceros (Table 71-2). The hymenal membrane and cervix are uniquely shaped anatomic structures in the rhinoceros (see Fig. 71-1).19,46,47,59
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree