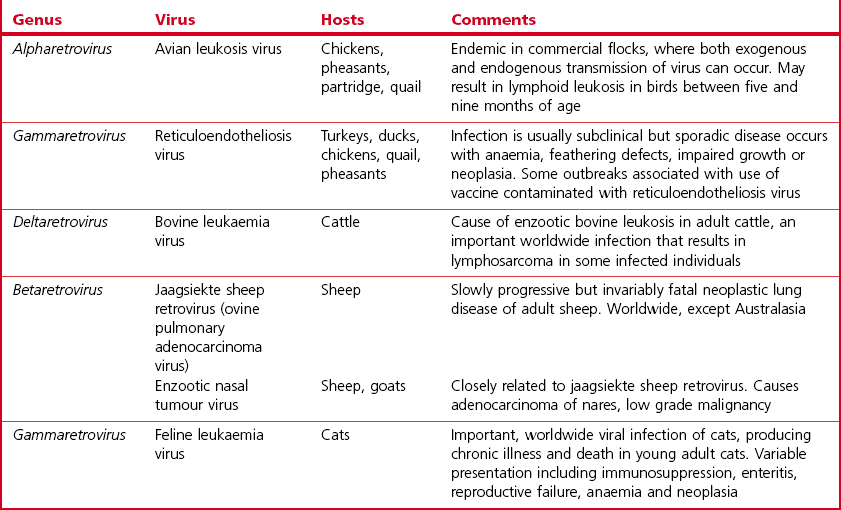
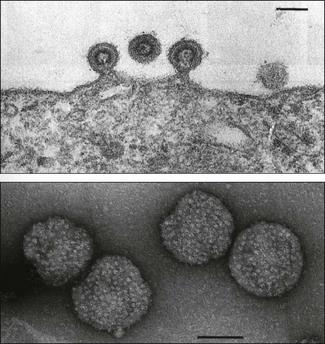
Chapter 65 Retroviruses (Latin retro, backwards) are spherical, labile, enveloped RNA viruses with a diameter of 80 to 100 nm (Fig. 65.1). Two subfamilies comprising seven genera are currently recognized within the family (Fig. 65.2): Alpharetrovirus, Betaretrovirus, Gammaretrovirus, Deltaretrovirus, Epsilonretrovirus and Lentivirus in the subfamily Orthoretrovirinae while the subfamily Spumaretrovirinae contains a single genus, Spumavirus. The family name refers to the presence in the virion of the enzyme reverse transcriptase which is encoded by the pol gene. The envelope is acquired from the plasma membrane of the host cell. It surrounds an icosahedral capsid which contains two identical linear, positive-sense, single strands of RNA and a number of core proteins including the enzymes reverse transcriptase and integrase. Historically, based on their appearance using electron microscopy, retroviruses were described as A-type, B-type, C-type and D-type particles. Retroviruses are sensitive to heat, lipid solvents and detergents. However, on account of their diploid genomes, they are relatively resistant to UV light. Figure 65.1 Top: Budding of typical type C retrovirus virions; Bottom: Retrovirus virions stained negatively with uranyl acetate showing peplomers on their surface. Bars represent 100 nm. Reprinted with permission: Veterinary Virology Third Edition (1999). Murphy et al., Academic Press. Page 365. The important oncogenic retroviruses of veterinary species are presented in Table 65.1. The genus Epsilonretrovirus contains viruses associated with neoplasia in fish. Lentivirus (Latin lentus, slow) infections are characterized by diseases with long incubation periods and insidious protracted courses. Examples of important animal and human lentivirus diseases include acquired immunodeficiency syndrome (AIDS), feline immunodeficiency, equine infectious anaemia and maedi-visna (Table 65.2). Spumaviruses (Latin spuma, foam) have been described in primates, cattle, horses and cats. They cause vacuolation of cultured cells, but are not associated with clinical disease. Table 65.2 Lentiviruses of veterinary importance Both replication-competent and replication-defective retroviruses make up the avian leukosis virus (ALV) group. These viruses are associated with neoplastic conditions in chickens including lymphoid, erythroid and myeloid leukoses, fibrosarcoma, haemangiosarcoma and nephroblastoma. Lymphoid leukosis, a B cell lymphoma, is the most common and economically important. Viral envelope glycoproteins determine virus neutralization properties, viral interference patterns and host range. Avian leukosis viruses can be divided into ten subgroups (A to J) on the basis of differences in these glycoproteins. Subgroups A, B, C, D, E and J contain the isolates from chickens. Isolates from outbreaks of disease in chickens generally belong to subgroup A. Endogenous avian leukosis viruses, subgroup E, are commonly present in chickens and are transmitted vertically in the germline cells. Subgroup J isolates are associated with myeloid leukosis in broilers and have arisen from recombination of a novel family of endogenous viruses (ev/J) and exogenous avian leukosis viruses (Benson et al. 1998). • Post mortem findings and histopathological determination of tumour type are often sufficiently characteristic to be diagnostic. • Differentiation from Marek’s disease is important. It is based on the age of affected birds, the presence of bursal tumours, an absence of thickening of peripheral nerves and a histological assessment of neoplastic cell types. • Virus isolation is difficult and generally not attempted. • Commercial ELISA kits for the detection of ALV p27 group-specific antigen are available. However, ALVs are widespread in poultry flocks and may be present in the absence of tumour formation. Detection of ALVs in flocks is particularly useful in control programmes aimed at the elimination of ALV-positive birds from breeder flocks. • The presence of flock infection can be demonstrated by detecting antibodies in serum or egg yolk. Suitable assays include virus neutralization, ELISA and indirect immunofluorescence. • The RT-PCR assay has been developed for the detection of ALV (Smith et al. 1998, Cavanagh 2001). Sequencing of the amplicon can be used to determine the infecting subgroup and to differentiate endogenous ALV from exogenous ALV (Pham et al. 1999). Alternatively primers specific for particular subgroups may be used (Garcia et al. 2003, Silva et al. 2007). This worldwide, retroviral disease of adult cattle is characterized by persistent lymphocytosis and the development of B cell lymphosarcoma in a number of infected animals. A number of countries have eradicated enzootic bovine leukosis (EBL), while other countries are embarking on eradication programmes. Bovine leukaemia virus (BLV) is labile and intimately cell-associated. Transmission occurs by direct contact or transplacentally through the transfer of blood or secretions such as colostrum and milk containing infected lymphocytes. Iatrogenic transmission is important. Transplacental transmission is not particularly efficient with less than 10% of calves born to infected dams being infected at birth. In addition, calves are protected for several months by maternally derived antibody. Typically animals are infected between six months and three years of age (Hopkins & DiGiacomo 1997). The prevalence of infection is higher in dairy cattle than in beef cattle. Susceptibility to infection is influenced by the animal’s genotype. • Several serological tests such as AGID, ELISA and radioimmune assay can be used for the detection of antibodies to BLV, usually antibodies directed against the gp51 and p24 of the virus. Both indirect and blocking ELISAs are available commercially and may be designed for use with bulk milk samples or serum samples. Antibodies present in calves less than six months of age may be colostral in origin. • Virus isolation, by cultivation of peripheral blood lymphocytes, is not performed routinely. Infected cells are usually co-cultivated with an indicator cell line such as bovine lung cells and infectious virus production is encouraged by the use of mitogens. The virus causes syncytial development in the cell sheet. • The polymerase chain reaction has been utilized for the detection of provirus in peripheral blood lymphocytes (Ballagi-Pordany et al. 1992, Belak & Ballagi-Pordany 1993). It is useful as a confirmatory test in individual cases or as an adjunct to large-scale serological testing. Jaagsiekte (Afrikaaner word meaning ‘panting sickness’) is caused by jaagsiekte sheep retrovirus (JSRV), also known as ovine pulmonary adenocarcinoma virus. The disease is also called ovine pulmonary adenomatosis and is a slowly progressing neoplastic disease of adult sheep. With the exception of Australasia and Iceland, jaagsiekte has a worldwide geographical distribution. Infection occurs rarely in goats. About 20 copies of related endogenous betaretroviruses (enJSRV) occur in the genome of sheep and goats. Expression of these endogenous viruses during foetal ontogeny may be the reason for the apparent immune tolerance and lack of immune response in mature sheep to exogenous JSRV (Palmarini et al. 2004). Transmission of JSRV occurs by the respiratory route and close contact facilitates spread of infection. The disease incidence in an infected flock may be up to 20%, it is influenced by breed and the type of flock management. The incubation period is highly variable ranging from several months up to two years. Virus replication occurs in two types of pulmonary cells, type II pneumocytes and non-ciliated bronchial cells. Tumours arising from these cell types progressively replace normal lung tissue. A viral oncogene has not been detected and the mechanism of neoplastic transformation is unclear. Studies have confirmed the transformation potential of the envelope protein in sheep (Caporale et al. 2006). Affected animals are usually three to four years of age. They are in poor bodily condition and display mouth breathing, particularly after exercise. By raising the hind legs and lowering the head (wheelbarrow test), a clear fluid can be seen to flow from the nostrils. At any one time only a single animal in an infected flock may be clinically affected. The course of the disease may extend over weeks or months and secondary pasteurellosis is common.
Retroviridae
Virus
Hosts
Comments
Maedi-visna virus
Sheep
Lifelong infection with clinical signs in some infected animals. Signs of progressive respiratory disease and indurative mastitis in older sheep, rarely presents as progressive neurological disease
Caprine arthritis-encephalitis virus
Goats
Worldwide distribution, mostly seen in dairy herds. Lifelong infection associated with polyarthritis and indurative mastitis in adults; progressive nervous disease in kids
Bovine immunodeficiency virus
Cattle
Widely distributed but pathogenicity unclear
Equine infectious anaemia virus
Horses, donkeys, mules
Lifelong infection with recurring febrile episodes and anaemia
Feline immunodeficiency virus
Cats
Worldwide distribution. Lifelong infection characterized by persistent viraemia and immunosuppression in cats over five years of age
Avian leukosis
Diagnosis
Enzootic bovine leukosis
Diagnosis
Jaagsiekte
Pathogenesis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree