CHAPTER 30 Respiratory and Thoracic Medicine
The Upper Respiratory Tract
Clinical signs of upper respiratory tract disease, including sneezing and nasal discharge, are common in cats (Box 30-1). Some diseases are associated with sneezing, and others are more commonly associated with stertorous breathing, with or without gagging. Coughing can sometimes be present, as well as epiphora, halitosis, dysphagia, and nonspecific signs such as lethargy, inappetence, and weight loss.1,12,33 Laryngeal disease is rare in the cat but may present as acute or chronic dyspnea, stridor, dysphagia, and signs of upper airway obstruction.84 Common causes of upper respiratory disorders in cats include trauma, foreign bodies, infectious agents, brachycephalic syndrome, inflammatory polyps, tooth root infections or other oral disease, nasopharyngeal stenosis, chronic rhinosinusitis, and neoplasia.1,12,33 The most common causes of laryngeal disease in the cat are laryngeal paralysis and laryngeal neoplasia.92 A complete diagnostic workup is important to determine the etiology so that the treatment regimen can be appropriately directed and maximal response to therapy is obtained.76,77
Clinical Signs
Nasal Disease
Nasal discharge is the most common clinical sign associated with nasal disease and can be serous, mucopurulent, or hemorrhagic1,12,33 (see Box 30-1). Serous nasal discharge is characteristic of most acute diseases of the nasal cavity and may precede mucopurulent nasal discharge. If the serous nasal discharge is chronic, viral and allergic etiologies are most common. Mucopurulent nasal discharge implies inflammation and occurs in association with fungal disease, primary bacterial disease, or overgrowth of normal bacterial flora secondary to any chronic nasal disease, including neoplasia, chronic rhinosinusitis, oronasal fistula, foreign body, inflammatory polyp, fungal disease, and viral disease. In addition, cats with vomiting or regurgitation can develop sneezing or nasal discharge by aspirating gastrointestinal content into the nose through the nasopharynx.
Epistaxis alone is most common with trauma, acute foreign body, hypertension, and coagulopathy. Epistaxis that develops in conjunction with or after mucopurulent discharge is most common with fungal disease, neoplasia, oronasal fistula, and occasionally chronic foreign bodies. Vasculitis occurs in dogs with diseases such as ehrlichiosis and bartonellosis but is rare in cats. Unilateral nasal discharge is more likely with foreign bodies, oronasal fistula, and neoplasia, although the latter can become bilateral as it progresses. Bilateral discharge is nonspecific and can be found with almost any etiology.33
Stertor is a harsh, audible snoring sound associated with inspiratory breathing. Cats that experience stertor while awake are also likely to snore when sleeping. Stertor indicates airway obstruction and is most common with such conditions as nasopharyngeal polyps, nasopharyngeal stenosis, and neoplastic masses that occlude the airway. It may also occur as a result of airway occlusion caused by turbinate inflammation. Facial deformity is relatively uncommon but is usually associated with neoplastic processes and fungal infections, particularly Cryptococcus spp.1
Laryngeal Disease
Laryngeal disease is rare in the cat but may present as acute or chronic dyspnea, signs of upper airway obstruction, stridor (a harsh, high-pitched sound heard on inspiration), and dysphagia. Coughing or gagging may also be appreciated, and aphonia (loss of voice) or a change in voice has been reported.84
General Diagnostics
Signalment and lifestyle will often help refine the differential list and direct a diagnostic workup. Brachycephalic breeds may be predisposed to nasal disorders because of their physical conformation.85,112 Neoplasia is more likely in older cats,33 and nasopharyngeal polyps are more common in younger cats.40 Cats with outdoor access are more likely to develop foreign bodies, trauma, or infectious etiologies.33 Cats in crowded housing conditions such as catteries, shelters, and multicat households are more likely to develop acute or chronic viral or bacterial rhinitis.32 Obtaining a complete history is important for determining the duration of the clinical signs. Acute onset of clinical signs is common with viral agents, foreign bodies, and trauma. The diagnostic workup of sneezing and nasal discharge is commonly completed in three phases (Box 30-2).
BOX 30-2 Staged Workup of Upper Respiratory Disease
Phase 1: Noninvasive Tests
Most cats with acute disease are generally evaluated with noninvasive tests and therapeutic trials. A complete physical examination with careful attention to the head and neck should be performed, including ocular retropulsion. Firm resistance to retropulsion of the orbit or a painful reaction could be indicative of a retrobulbar lesion. Otic examination should be completed to evaluate for bulging or discoloration of the tympanum; these changes commonly occur with nasopharyngeal polyps. Deformation of the nose or face, exophthalmia, or pain on palpation of the nasal or facial bones is most consistent with fungal disease or neoplasia.1 Oral examination should be performed to assess for dental disease that could be causing an oronasal fistula, gingivostomatitis that could be consistent with feline herpesvirus (FHV-1) or feline calicivirus (FCV) infections, and defects in the hard or soft palate. External ocular examination may reveal conjunctivitis that could indicate FHV-1, FCV, Mycoplasma spp., or Chlamydophila felis infections. Fundic examination is performed to evaluate for lesions consistent with lymphoma or Cryptococcus neoformans infection. A cold microscope slide can be placed in front of the nose to assess airflow and may aid in determining if disease is unilateral or bilateral, although this should not limit diagnostic investigation to the obstructive side of the nose because bilateral disease may be present.
If lymph nodes draining the head are enlarged, they should be aspirated to evaluate for the presence of lymphoma, metastatic neoplasia, and fungal agents. Bacterial culture and antimicrobial susceptibility testing on nasal discharges are generally not recommended because results are difficult to interpret in that they typically yield normal intranasal bacterial flora.43 However, in respiratory outbreaks in catteries, pet stores, shelters, and multicat households, culture may be indicated to determine whether a pathogenic Bordetella bronchiseptica isolate is present.
Molecular diagnostic assays are now available for many respiratory agents, including FHV-1, FCV, C. felis, Mycoplasma spp., and B. bronchiseptica. However, cats can be asymptomatic carriers of these agents, and the FHV-1, FCV, B. bronchiseptica, and C. felis assays also amplify vaccine strains of the organisms, which means that positive results do not prove a disease association. This is especially true for FHV-1 and FCV, which may have a relatively high prevalence in the healthy cat population.52,78,98 Recently, a study failed to link Bartonella spp. infection to rhinitis in cats; therefore at this time recommendations to perform Bartonella spp. serology, culture, or polymerase chain reaction (PCR) assays in cats with upper respiratory tract signs are controversial.6 If a clinician chooses to test for evidence of Bartonella spp. infection, the cat should be evaluated by serology and PCR or culture because serology alone has been shown to yield false-negative results in up to 15% of infected cats.8 In addition, because only approximately 40% of seropositive cats are currently infected, a positive serologic test result does not prove bartonellosis.8 See Chapter 33 and the subsequent sections in this chapter about individual agents for a further discussion of molecular assays.
A complete blood cell count (CBC), serum biochemical panel, and urinalysis is recommended to rule out other systemic disease processes in cats with chronic disease. In general, results of the CBC are of low yield but may reveal eosinophilia in some cats with fungal or allergic disease, thrombocytopenia in some cats with epistaxis, or other cytopenias that might accompany feline leukemia (FeLV) or feline immunodeficiency virus (FIV) infections. FeLV and FIV do not cause sneezing and nasal discharge primarily, but they have been associated with lymphoma and may induce immunodeficiency that predisposes to other infections; therefore testing for these agents is indicated. A Cryptococcus antigen test is also recommended as a preliminary test for any cat with chronic nasal discharge, but particularly for those with nasal deformation, lymphadenopathy, or retinal lesions.59 Although thoracic radiographs are generally normal, they are still indicated to rule out pulmonary involvement of fungal disease and metastatic neoplasia. In cats with epistaxis, blood pressure measurement, coagulation profile, and buccal mucosal bleeding test are recommended, and thromboelastography may also be useful.
Phase 2: Imaging, Biopsy, Deep Cultures
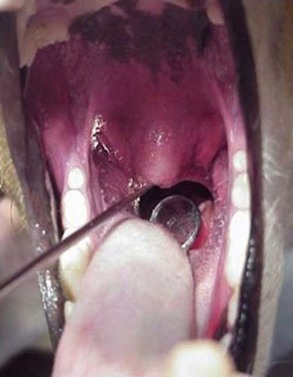
General anesthesia is induced by administering approximately one third of an induction dose of propofol (4 to 6 mg/kg intravenously), a short-acting thiobarbiturate, or ketamine combined with diazepam (ketamine 5 mg/kg intravenously and valium 0.3 mg/kg intravenously). The arytenoids are examined before intubation to make sure both are abducting normally on inspiration. Dopram can be used to stimulate respiration and increase intrinsic laryngeal motion at a dose of 2.2 mg/kg, administered intravenously. Oropharyngeal examination is performed to evaluate thoroughly for masses, foreign bodies, or palate defects. A spay hook and dental mirror can be used to help manipulate the soft palate to a position allowing visualization of the nasopharynx so that polyps, other masses, foreign material, or nasopharyngeal stenosis can be checked (Figure 30-1). A thorough dental examination should be performed and all teeth probed for evidence of oronasal fistula.
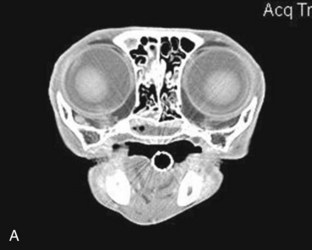
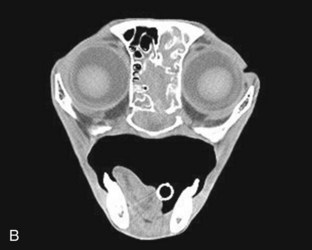
If a definitive diagnosis is not made, a CT scan or nasal, sinus, and dental radiographs are performed. If radiographs are performed, anesthesia is required for accurate positioning and should include a lateral view, ventrodorsal view, and intraoral and open-mouth bullae views. Nasal imaging can reveal increased density in the nasal cavity or bony lysis that could be consistent with a mass, turbinate destruction consistent with chronic rhinosinusitis or fungal disease, as well as radio-opaque foreign objects or tooth root abscessation.44,69 Although more expensive and not widely available, a CT scan has the added advantage of better visualization of the sinuses and tympanic bullae and better assessment of bony lysis; it also allows assessment of the cribriform plate and brain so that the extent of a lesion can be evaluated86 (Figure 30-2). It is also faster to perform than a full series of skull radiographs and allows for radiotherapy treatment planning if indicated. It is the preferred imaging modality, especially if a mass is suspected. Imaging should be performed before rhinoscopy and biopsy to prevent hemorrhage from obscuring details in the nasal passages.
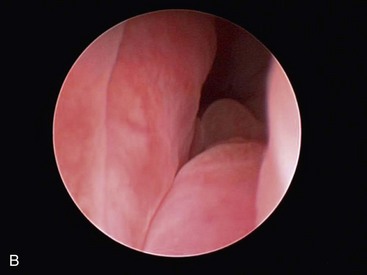
Depending on imaging findings, the nasopharynx is examined with a flexible rhinoscope, and rigid rhinoscopy of the anterior nasal cavity is then performed (Figure 30-3). Rhinoscopy allows direct visualization of the nasal cavity, detection and removal of foreign objects, detection and débridement of fungal plaques, as well as assessment for inflammation, turbinate destruction, and masses. However, should a mass be present, rhinoscopy does not allow assessment of the extent of bony lysis (hence the importance of additional imaging). In addition, because gross appearance of the nasal mucosa on rhinoscopy does not always correlate with histopathologic diagnosis, biopsies should always be performed.37
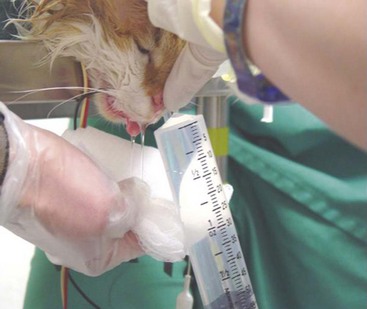
If no foreign material is visualized on rhinoscopy, the nasal cavity is flushed with sterile saline to evaluate for the presence of hidden material. The cuff of the endotracheal tube should be checked for full inflation before performing nasal lavage with saline administered under pressure. In cats lavaging should be performed from the anterior nares caudally. Gauze should be placed in the oropharyngeal area and then a 20-, 35-, or 60-mL syringe can be used to forcefully flush saline through the nose while the nares are being pinched off to create pressure (Figure 30-4). Material flushed from the nose (or oropharynx) should be caught on the gauze and examined for foreign objects. If no foreign material is located, biopsies are then made using a bone curette or the largest biopsy instrument that can be passed through the nares. Most rigid endoscopes are too large for the biopsy sleeve to be used in many cats; a gastroscopic biopsy instrument can often be passed next to the camera of a rigid scope to perform directed biopsies. Alternatively, the biopsy site can be directed by the results of diagnostic imaging or by rhinoscopy. If indicated, bacterial and fungal cultures are made using material from flush or biopsied tissues.39
Disease-Specific Recommendations: The Nasal Cavity
Anatomic and Functional Disorders
Nasopharyngeal Polyps
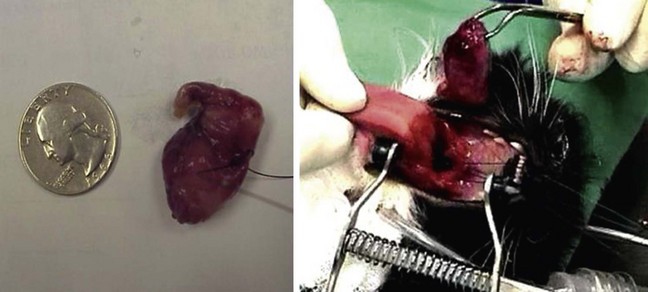
Nasopharyngeal polyps are non-neoplastic, inflammatory nodules that occur most commonly in young cats. They originate in the middle ear or auditory canal and can grow out through the nasopharynx or, alternatively, the tympanum.34,40 Why the growths occur is unknown, but because they tend to occur when the cat is young, a congenital etiology has been postulated.2 The possible association of polyps with infectious agents has also been explored, including FHV-1, FCV, C. felis, Mycoplasma spp., and Bartonella spp.,41,108 but to date no organism has definitely been proven to be a cause. Large polyps can be detected by palpation through the soft palate, and otic examination may reveal discoloration or bulging of the tympanum. When extending into the nasopharynx, polyps disrupt the normal flow of secretions, resulting in secondary bacterial infections, mucopurulent nasal discharge, stertorous breathing, and gagging. Signs of middle ear involvement, such as Horner’s syndrome and head tilt, can also be seen. Diagnosis can be confirmed with examination of the nasopharynx under sedation with a dental mirror and spay hook or rhinoscope, as previously described. A bulla radiography series or CT scan should be performed to determine whether there is bulla involvement. However, if there is no evidence of middle ear–associated clinical disease and the polyp can be removed by way of the mouth, many clinicians will perform removal using traction and wait for a recurrence before performing a bulla osteotomy on account of the high incidence of morbidity associated with bulla osteotomy40,108 (Figure 30-5). Complications of this procedure include Horner’s syndrome, facial nerve paralysis, and discomfort, and the recovery period is similar to that for a relatively invasive surgery. Without bulla osteotomy approximately 30% will be recurrent.108 However, combining removal by traction with a tapering course of glucocorticoids (1 to 2 mg/kg per day, by mouth, for 14 days followed by a taper dosage over the next 2 weeks) may improve the success rate.64 Bulla osteotomy is an effective surgical treatment, and when it is performed at initial presentation or at recurrence, most cases generally experience complete resolution.40,103,108
Brachycephalic Syndrome
Cats with brachycephalic conformation may experience difficulty with airflow due to the severe malformation of their nasal passages and nares, and potentially could be predisposed to nasal disease. A recent CT study of brachycephalic cats documented some of the abnormalities associated with this condition. It was found that the greater the degree of brachycephalia, measured by the amount of dorsal rotation of the maxillary canine tooth, the narrower the nasal cavity, nasal passages and nares.85 Stenotic nares also serve to limit inspired airflow. This condition may be improved by alar fold excision, performed with a laser or scalpel technique or alternatively a punch resection alaplasty.104 Nasopharyngeal turbinates have also been documented in brachycephalic cats and may serve to further reduce airflow through the nasopharyngeal area.25 Little information is available regarding surgical options for nasopharyngeal turbinates.
Nasopharyngeal Stenosis
Nasopharyngeal stenosis is a rare condition that involves narrowing of the choanae to the extent that little air is able to pass. This can occur as a result of chronic infections, aspiration rhinitis, or congenital defect.33,96 Clinical signs typically include stertorous, labored breathing and, less typically, nasal discharge. Diagnosis is determined by retroflex rhinoscopic assessment of the nasopharynx. In the past manual dilation and advanced surgical procedures combined with steroid therapy were the only therapeutic options, and recurrence was common.33 More recently, stenting of the nasopharynx has been described as a successful palliative measure.5
Infectious Disorders
Bacterial Agents
Diagnosis
Almost all cats with mucopurulent or purulent nasal discharge have a bacterial component to their disease. The bacterial agents that have been described as primary respiratory pathogens in cats include B. bronchiseptica, C. felis, Streptococcus canis, and Mycoplasma spp. However, Corynebacterium spp., Escherichia coli, Pasteurella multocida, Pseudomonas aeruginosa, Streptococcus viridans, and Staphylococcus intermedius are also commonly detected but generally thought to be secondary invaders.* Culture of either nasal flush samples or tissue biopsy samples yields similar species results, but aerobic and anaerobic cultures of nasal flushes were positive significantly more often in one study.39 Culture of nasal biopsies may be more representative for deep mucosal infections,38 but this has not been definitively shown. In another study different organisms were isolated from each collection technique, so it may be most complete to culture both nasal flush and biopsy samples.38 However, it should be remembered that positive culture results may not correlate with the cause of the disease on account of the presence of normal flora and other superficial bacteria.
Although B. bronchiseptica is a well-defined primary pathogen in dogs, the organism can be isolated from many clinically normal cats.32 Thus the positive predictive value (PPV) of serologic test results, culture, and PCR assay is low in cats. Many cats have antibodies against B. bronchiseptica, the organism is commonly cultured from cats in crowded environments, and there are sporadic reports of severe lower respiratory disease caused by bordetellosis in kittens and cats in crowded environments or other stressful situations.7,110 The organism was cultured on necropsy from the lower airways of several cats from shelters in Colorado, and in one shelter the organism was cultured from 19 of 40 cats (47.5%) with upper respiratory disease.91 However, the significance of infection in otherwise healthy pet cats appears to be minimal. For example, in client-owned cats in north central Colorado, the organism was rarely cultured from cats with rhinitis or lower respiratory disease (approximately 3%).109 B. bronchiseptica is easily grown, and culture is superior to PCR for this agent because antimicrobial susceptibility testing can be performed on isolates. Because the organism is not usually eliminated by treatment, follow-up culture or PCR assay after treatment has minimal benefit.9
C. felis is a common differential diagnosis for cats with clinical evidence of conjunctivitis and rhinitis; it is not a common cause of lower airway disease. The organism is difficult to culture, so PCR detection of microbial DNA from conjunctival swabs can be useful clinically. Because of the intracellular nature of the organism, adequate cellular material must be obtained from the conjunctival swab for analysis.28 PCR assay results can be used to prove a cattery has been cleared of the infection after treatment.95 Most, but not all, PCR-positive cats are clinically ill (e.g., 3.3% if healthy cats were positive in one study).13
Mycoplasma spp. are normal commensal organisms of the mucous membranes of multiple species, including cats. M. felis has been associated primarily with conjunctivitis but is suspected as a primary cause of rhinitis in cats as well.31,38,39 There are multiple Mycoplasma spp. of cats, and the pathogenic potential for most is unknown. If other primary diseases are present, even nonpathogenic Mycoplasma spp. may be associated with the disease process. Mycoplasma spp. culture can be difficult and takes longer than routine culture, and antimicrobial susceptibility is not provided by most laboratories. Culture of nasal biopsy samples rather than nasal flush samples may increase yield.39 Mycoplasma spp. PCR assays have at least some clinical utility, with some assays allowing for speciation, which is helpful in assessing the pathogenic potential of the organism. However, because Mycoplasma spp. are common flora, the PPV of the assays is likely to be low. Because the organism is not usually eliminated by treatment, follow-up culture or PCR assay after treatment has minimal benefit.
Treatment
If primary bacterial infections are suspected, doxycycline 10 mg/kg, administered orally once daily for cats with rhinitis with or without conjunctivitis, is usually effective (Table 30-1). Doxycycline is the treatment of choice for B. bronchiseptica, Mycoplasma spp., and C. felis infections,19,28 and in the last has been shown to be superior to topical administration of tetracycline.90 Side-effects in young kittens are less of a concern with doxycycline than tetracycline but should still be taken into consideration. Amoxicillin–clavulanate is a good choice in young animals and is effective for most organisms, with the exception of Mycoplasma spp. because these organisms lack a cell wall. Pradofloxacin has been shown to have efficacy against Mycoplasma spp.16,31 Enrofloxacin has also been shown to be effective for C. felis23 but should be used with caution in young cats because of possible adverse effects on cartilage. Although the drug has not been shown to damage chondrocytes in cats, this does occur in several other species. Clindamycin penetrates bone and tissues well and has an excellent anaerobic spectrum. Administering the liquid form of this drug is generally well tolerated if given cold. Azithromycin therapy (15 mg/kg, administered orally once daily) can be tried for cats with suspected resistant bacterial infections.*
TABLE 30-1 Pharmacologic Treatment of Upper Respiratory Tract Disease
| Class | Drug | Dosage |
|---|---|---|
| Antibiotics | Amoxicillin | 10-22 mg/kg, PO, every 12 hours |
| Amoxicillin-clavulanate | 13.75 mg/kg, PO, every 12 hours | |
| Azithromycin | 15 mg/kg, PO, every 24 hours | |
| Cefadroxil | 22 mg/kg, PO, every 12 hours | |
| Cephalexin | 22 mg/kg, PO, every 8 hours | |
| Chloramphenicol | 10-15 mg/kg, PO, every 12 hours | |
| Clindamycin | 10-12 mg/kg, PO, every 24 hours | |
| Doxycycline | 10 mg/kg, PO, every 24 hours | |
| Enrofloxacin | 2.5-5 mg/kg, PO, every 24 hours | |
| Marbofloxacin | 2.5-5 mg/kg, PO, every 24 hours | |
| Metronidazole | 10-15 mg/kg, PO, every 12 hours | |
| Orbifloxacin | 2.5-5 mg/kg, PO, every 24 hours | |
| Pradofloxacin | 5-10 mg/kg, PO, every 24 hours | |
| Trimethoprim-sulfonamide | 15 mg/kg, PO, every 12 hours | |
| Antihistamines | Cetirizine | 2.5-5 mg/cat, PO, every 24 hours |
| Chlorpheniramine | 2 mg/cat, PO, every 12 hours | |
| Clemastine | 0.68 mg/cat, PO, every 12 hours | |
| Fexofenadine | 5-10 mg/cat, PO, every 12 to 24 hours | |
| Hydroxyzine | 5-10 mg/cat, PO, every 8 to 12 hours | |
| Loratadine | 5 mg/cat, PO, every 24 hours | |
| Antifungals | Deoxycholate amphotericin B | 1) IV: 0.1-0.5 mg/kg: M, W, F; to 16 mg/kg total cumulative dose 2) SC: 0.5-0.8 mg/kg in 400 mL of 0.45% saline/2.5% dextrose; M, W, F; to 16 mg/kg total cumulative dose |
| Fluconazole | 50 mg/cat, PO, every 12 to 24 hours | |
| Itraconazole | 10 mg/kg, PO, every 24 hours | |
| Liposomal amphotericin B | 1 mg/kg IV; Mon, Wed, Fri; to 12 mg/kg total cumulative dose | |
| Antivirals | Cidofovir topical (0.5%) | 1 drop OU, every 12 hours |
| Famciclovir | 62.5 mg/cat, PO, every 12 hours, 14 days | |
| Interferon-alpha | 10 U PO, every 24 hours (chronic); 10,000 U SC, every 24 hours, 21 days (acute) | |
| Lysine | 500 mg/cat, PO, every 12 hours | |
| NSAIDs | Meloxicam | 0.025-0.1 mg/kg, PO, every 2 to 3 days |
| Piroxicam | 0.3 mg/kg, PO, every 2 days | |
| Glucocorticoids | Beclomethasone (inhaled) | 1-2 puffs, every 12 to 24 hours |
| Fluticasone (inhaled) | 1-2 puffs, every 12 to 24 hours | |
| Methylprednisolone acetate | 5-15 mg IM, every 3 to 4 weeks, as needed | |
| Prednisolone | 2.5-5 mg/cat, PO, every 1 to 2 days |
PO, By mouth; IV, intravenously; SC, subcutaneously; OU, each eye; NSAIDs, nonsteroidal antiinflammatory drugs; IM, intramuscularly.
Doxycycline and clindamycin have been associated with esophagitis and esophageal strictures in cats4,24,61 because of the poor secondary esophageal contractions in this species. The authors recommend never administering dry pills or capsules to cats. Drugs should be compounded into a liquid, administered, and then followed with a 3- to 6-mL liquid bolus or food, administered coated with butter or a product such as Nutri-Cal, or administered in a pill-delivery treat.27,50,111 Cats with acute disease are treated for 7 to 10 days, except for C. felis, in which 28 days of therapy is needed to eliminate infection.31,95 Chronic bacterial disease may require treatment for 6 to 8 weeks to adequately clear the infection if osteomyelitis exists. Pulse therapy may help some chronically affected cats but may induce antimicrobial resistant bacteria in other cats. Most cases of bacterial rhinitis are secondary to other diseases, including trauma, neoplasia, inflammation induced by viral infection, foreign bodies, inflammatory polyps, chronic rhinosinusitis, and tooth root abscessation. Thus if routine antibiotic therapy fails, a diagnostic workup should be performed.
Prevention
The currently available B. bronchiseptica vaccine for intranasal administration can be administered as early as 4 weeks of age, has an onset of immunity as early as 72 hours, and has a minimum duration of immunity of 1 year.81 The American Association of Feline Practitioners (AAFP) Feline Vaccine Advisory Panel, and the European Advisory Board on Cat Diseases (ABCD) recommendations suggest that Bordetella vaccination should be considered primarily for use in cats at high risk for exposure and disease, such as those with a history of respiratory problems and living in humane shelters with culture-proven outbreaks.19,81 However, because the vaccine is administered by the intranasal route, mild sneezing and coughing can result, which may influence case management of kittens housed in shelters or humane societies. Because the disease is apparently not life-threatening in adult cats, is uncommon in pet cats, responds to a variety of antibiotics, and is considered minimally zoonotic,18 routine use of this vaccine in the majority of client-owned cats seems unnecessary.
Killed and modified live C. felis–containing vaccines are available. In recent studies C. felis was amplified from conjunctival swabs of 3.2% of cats with conjunctivitis49 but 0% of nasal discharges from cats housed in a humane society.91 FVRCP vaccines that also contained C. felis were associated with more vaccine reactions in cats when compared to other products.63 Because infection of cats by C. felis generally results only in mild conjunctivitis, is easily treated with antibiotics, has variable prevalence rates, and is of minimal zoonotic risk to humans, some researchers have questioned whether C. felis vaccination is ever necessary in the United States.14 Duration of immunity for Chlamydophila vaccines may be short-lived, so high-risk cats, such as those in multicat environments or where there is a history of chlamydial infection, should be immunized before a potential exposure.
Viral Agents
Diagnosis
The most common viruses associated with feline respiratory disease are FCV and FHV-1. Both viruses are extremely common in cats, particularly those from crowded environments such as pet stores, catteries, and shelters.14,32 There are many strains of FCV, and mutations resulting in new strains are common. This organism is a common differential diagnosis for cats with clinical evidence of rhinitis, stomatitis, oral ulceration, and conjunctivitis (Figure 30-6). Less commonly, FCV is associated with polyarthritis, lower airway disease in kittens, and virulent systemic disease.78 Some variants of FCV are thought to induce systemic vasculitis in cats (virulent systemic calicivirus [VS-FCV]), and clinical signs can be severe even in cats previously vaccinated with FVRCP vaccines.10,36,72,87,105 VS-FCV strains arise spontaneously from endogenous FCV strains, and outbreaks have resolved quickly after the initial cases were recognized. Currently, it is unknown how often these outbreaks occur and whether the number of outbreaks is increasing. The VS-FCV strains evaluated to date have been genetically and antigenically diverse.42,71
Virus isolation can be used to document current infection but takes at least several days for results and is not performed by all laboratories. Because of widespread exposure and vaccination, the PPV of serologic tests is poor. Reverse transcriptase (RT) PCR assays can be used to amplify the RNA of calicivirus, and results can be made available quickly. However, these assays also amplify vaccine strains of FCV (Lappin MR: unpublished data, 2010). FCV RNA can be amplified from samples collected from normal carrier cats as well as clinically ill cats, and PCR assays therefore have poor PPV. In addition, amplification of FCV RNA cannot be used to prove virulent systemic calicivirus infection.78 False-negative results of FCV RT-PCR can also occur if inadequate RNA is present on the submitted swab or if the organism has been cleared to levels below the sensitivity limits of the assay by specific immune responses. Because treatment does not eliminate FCV infection, there is no benefit to follow-up culture or RT-PCR testing.
FHV-1 is a common differential diagnosis for cats with clinical evidence of rhinitis, stomatitis, conjunctivitis, keratitis, and facial dermatitis. Because of widespread exposure and vaccination, the PPV of serologic tests is poor. FHV-1 infection can be documented by direct fluorescence staining of conjunctival scrapings, virus isolation, or PCR.98 FHV-1 DNA can be amplified from conjunctival cells of approximately 20% of healthy cats; therefore the PPV of PCR assays for this agent is low.79 Currently used PCR assays also detect vaccine strains of FHV-1, further lessening the PPV.52 Quantitative PCR may ultimately prove to correlate with the presence or absence of disease but failed to correlate to presence of conjunctivitis in one small study in the authors’ laboratory.49 The negative predictive value of the FHV-1 PCR assays is also in question because many cats that are likely to have FHV-1–associated disease are PCR negative. This may relate to clearance of FHV-1 DNA from tissues by the immune reaction. Tissue biopsies have greater sensitivity than conjunctival swabs but do not necessarily have greater predictive value.93 FHV-1 DNA can be amplified from the aqueous humor of some cats, but whether this indicates FHV-1–associated uveitis is unknown.54 Because treatment does not eliminate FHV-1 infection, there is no benefit to follow-up culture or PCR testing.
Treatment
Therapy for FCV consists mainly of supportive care, which is often needed for cats with VS-FCV infections, and may consist of intravenous fluids, antibiotics for concurrent bacterial infections, and interferon. Interferon may augment immune responses to viral infections by upregulating key cytokines.101 Feline interferon omega (1 to 2.5 million IU/kg, intravenously or subcutaneously, every 24 to 48 hours for up to 5 doses, then reduce to twice weekly and eventually to once weekly, depending on clinical response) (Virbagen Omega, Virbac Animal Health) inhibits FCV replication in vitro, but results of controlled studies evaluating efficacy in clinically affected cats with respiratory disease are not available. If human alpha interferon is used systemically for cats with life-threatening FCV or FHV-1 infections, 10,000 U/kg, administered subcutaneously, can be administered safely once daily, but controlled data concerning efficacy are not available. Recently, use of feline interferon therapy has been used to improve quality of life in cats with FeLV and FIV infections.11 In one study low-dose oral interferon therapy (10 U/kg, orally, daily alternating 7 days on, 7 days off, for 6 months) improved quality of life in cats with FIV infections.73 The effect of oral interferon is thought to be from mediation of inflammatory cytokines. There may also be effects against chronic FHV-1 or FCV infections, but controlled data are not available. Topical administration of alpha interferon in saline to the eyes of cats with conjunctivitis or the nose has been recommended by some veterinarians as an aid in the management of some cats with acute or chronic FHV-1 or FCV infections.
Recently, antiviral drugs have become more popular in the management of cats with acute or chronic FHV-1 infections. Currently available antiviral medications are efficacious only for DNA viral infections such as FHV-1, and not RNA viruses such as FCV, because they interfere with viral DNA synthesis and thus viral replication. Acyclovir and valacyclovir have been administered to some cats but can induce bone marrow suppression and are minimally effective for FHV-1; therefore they should no longer be used.51,66 Famciclovir is safe and effective and is used for both acute and long-term therapy for cats with FHV-1 infections. One dose that has been used with apparent clinical efficacy is 62.5 mg, orally, every 12 hours for 14 days.58 However, recent pharmacokinetic studies indicate that higher doses may be needed for activity against FHV-1.99
Idoxuridine and trifluridine have been used topically in cats with conjunctivitis or keratitis resulting from FHV-1 infection, but these must be administered multiple times per day and are irritating. Recently, cidofovir was used in a small experimental FHV-1 conjunctivitis study and was shown to lessen clinical signs and FHV-1 shedding.21 Lysine at 250 to 500 mg, orally, every 12 hours may be helpful in some cats with acute or chronic rhinosinusitis caused by FHV-1 infection (not FCV).53 However, in several controlled studies of cats fed a lysine-fortified diet, a significant positive effect was not noted.17,55,80
Intranasal administration of modified live FHV-1 and FCV vaccines may lessen disease in some chronically infected cats, but controlled data are lacking. If there is a positive response to intranasal vaccination in a cat with chronic disease, this form of immunotherapy can be administered up to three times per year. Intranasal vaccination has been shown to potentiate cell-mediated immunity to FHV-1 better than parenteral vaccination.48 Chronic administration of one commercially available probiotic (FortiFlora, Purina Veterinary Diets) was shown to enhance T-helper lymphocyte numbers in cats.106 When this probiotic was administered to cats with chronic FHV-1 infections that were then subjected to mild stress, improved conjunctivitis scores were noted in some of the cats in the treatment group.47 See the section on chronic rhinosinusitis for a discussion of other nonspecific therapies.
Prevention
Specific pathogen-free (SPF) cats inoculated with one dose of intranasal modified live FVRCP vaccine had significantly less clinical signs than control cats as soon as 4 days when challenged with virulent FHV-1 in one study.45 Administration of the intranasal FVRCP vaccine was also shown to induce FCV antibody responses in SPF kittens more quickly than a modified live FVRCP vaccine administered parenterally.46 Thus the intranasal route of administration may be preferred for the primary or booster immunization of kittens housed in environments at high risk for exposure to FHV-1 or FCV, such as shelters, humane societies, catteries, boarding facilities, and multicat households. However, because administration of intranasal FHV-1 and FCV vaccines can induce transient mild sneezing or coughing, the owners should be informed of these potential side effects. Additionally, these vaccine side effects may influence case management of kittens housed in shelters or humane societies. Subcutaneous vaccines are recommended if concerns about the respiratory side effects of intranasal vaccines exist. Currently, yearly revaccination for cats in high-risk environments (e.g., shelters, catteries, multicat household) and a 3-year revaccination interval for cats in low-risk environments (indoor-only with no contact with other cats) are recommended.78,81,98
Inactivated vaccines containing VS-FCV are now available (Calicivax, Boehringer Ingelheim Vetmedica). The product contains a traditional FCV vaccine strain as well as a VS-FCV strain. Cross-neutralization studies show that cats inoculated with more than one FCV strain inactivate more FCV strains in vitro than cats inoculated with one FCV strain.74,75 In addition, a recent challenge study illustrated that kittens vaccinated with the dual-strain product were protected from clinical signs of VS-FCV.35
Fungal Agents
C. neoformans and Aspergillus spp. are the most common causes of respiratory tract fungal infection in cats.3,57,68 Cryptococcosis is most common and should be considered a differential diagnosis for cats with respiratory tract disease, subcutaneous nodules, lymphadenopathy, intraocular inflammation, fever, and central nervous system disease.57 Infected cats range from 6 months to 16 years of age, and male cats are overrepresented in some studies.67 Infection of the nasal cavity is reported most frequently and commonly results in sneezing and nasal discharge. The nasal discharge can be unilateral or bilateral, ranges from serous to mucopurulent, and often contains blood. Granulomatous lesions extruding from the external nares, facial deformity over the bridge of the nose, and ulcerative lesions on the nasal planum are common (Figure 30-7). Submandibular lymphadenopathy is detected in most cats with rhinitis. Definitive diagnosis of cryptococcosis is based on antigen testing or cytologic, histopathologic, or culture demonstration of the organism.
Cats with cryptococcosis have been treated with amphotericin B, ketoconazole, itraconazole, fluconazole, and 5-flucytosine alone and in varying combinations. Responses have varied among studies, but good to excellent treatment responses are often achieved in cats administered fluconazole or itraconazole.60,67 Because of toxicity and the availability of more efficacious drugs, ketoconazole is no longer recommended by these authors. Fluconazole at 50 mg per cat, orally, once or twice daily is recommended because it results in the fewest side effects, has the best penetration across the blood–brain and blood–ocular barriers of the azoles, and has been shown to have good efficacy.60,67 If life-threatening infection is occurring or the cat is failing to respond to an azole drug, amphotericin B should be used.67 A typical amphotericin protocol involves intravenous infusions on a Monday–Wednesday–Friday schedule until a cumulative dose of 16 mg/kg has been reached. Nephrotoxicity is the most serious side effect, and an initial infusion dose of 0.1 mg/kg is used as a test dose. The dose can be slowly increased to 0.5 mg/kg if it is well tolerated clinically and if renal values remain stable.26,56 A successful subcutaneous protocol has also been described in which 0.5 to 0.8 mg/kg is added to 0.45% saline/2.5% dextrose, and the total volume is given two to three times weekly to a cumulative dose of 8 to 26 mg/kg.56,67
In addition to the deoxycholate form of amphotericin B, a liposomal formulation is also available. It is thought that less nephrotoxicity is seen with the liposomal product, and a recommended dose regimen is 1 mg/kg, intravenously, on a Monday–Wednesday–Friday schedule until a cumulative dose of 12 mg/kg has been reached.26 Focal nasal and cutaneous cryptococcosis generally resolves with treatment; central nervous system, ocular, and disseminated diseases are less likely to respond to treatment.60,67 Treatment should be continued for at least 1 to 2 months past resolution of clinical disease, or until antigen titers are negative.20,67 People and animals can have the same environmental exposure to Cryptococcus spp., but zoonotic transfer from contact with infected animals is unlikely.
Aspergillosis is less common than cryptococcosis but can be equally devastating.3,102,112 Clinical signs of mild disease are similar to those of nasal cryptococcosis. Sino-orbital aspergillosis was recently described in cats and appears to be more aggressive than canine aspergillosis, involving invasion into surrounding structures.3 Ocular involvement, such as exophthalmos and ocular discharge, can be seen in addition to nasal signs. Diagnosis of aspergillosis is based on visualization of fungal plaques on rhinoscopy or fungal hyphae on cytology or biopsy. Infection can be caused by either Aspergillus spp. or Penicillium spp., which can be difficult to differentiate cytologically. Fungal culture seems less sensitive and specific than visualization on rhinoscopy or biopsy.112 Therapy with oral itraconazole and fluconazole has been documented as 50% to 60% efficacious,102,112 and better efficacy with nasal clotrimazole therapy has been reported in a few cases.102
Chronic Inflammatory Disorders
Chronic Rhinosinusitis
Lymphocytic–plasmacytic, eosinophilic, and idiopathic rhinosinusitis are a constellation of diagnoses obtained by way of histopathology that are collectively referred to as chronic rhinosinusitis. In many cases this is a diagnosis of exclusion when other etiologies have been ruled out. This syndrome is one of the most significant causes of sneezing and nasal discharge in the cat.33 The nasal discharge is generally serous, but secondary bacterial infections can result in the development of mucopurulent nasal discharge (Figure 30-8), and inflammation can be severe enough to cause intermittent hemorrhage.33 The clinical signs can persist for years. Cats with relatively stable disease that encounter a sudden change in severity should be re-evaluated for the presence of a more severe secondary disease, such as fungal rhinitis or neoplasia.
There is a subset of chronic rhinosinusitis cats that have a history of acute FHV-1 or FCV upper respiratory infections at a younger age, and it is postulated that an early severe viral infection may trigger chronic disease.38 In addition, it is estimated that approximately 80% of cats are latently infected with FHV-1,22 and so another possible scenario for chronic viral rhinosinusitis is the presence of latent FHV-1 viral infections that are triggered into recrudescence by stressful events. In such cats with a prior history of viral infection, therapies such as lysine, antivirals, and immunomodulators are often tried, as previously discussed. Subjective improvement in clinical signs has been noted in response to cationic liposome DNA complexes (CLDC) immunomodulatory therapy in a small pilot study currently under way at Colorado State University, as well as in a previously published study.107 Stress is thought to play a role in the clinical severity and potential for recurrence of chronic viral rhinosinusitis, particularly if FHV-1 latency or chronic FCV infections are involved. Environmental measures to decrease stress, allocate resources in multicat households, and provide antianxiety therapies such as feline facial pheromone use (Feliway, Ceva Animal Health) may also provide some benefit. Controversy surrounds the use of immunosuppressive therapy in these patients: It may not be beneficial and runs the risk of exacerbating viral and bacterial components of the disease syndrome.
In many cases there is no history of viral infection or any other predisposing cause. Generally, idiopathic chronic rhinosinusitis is somewhat refractory to treatment, and palliation of clinical signs, rather than cure, is the goal of medical management. Broad-spectrum antibiotics are often prescribed to manage secondary infections. Administration of antihistamines such as chlorpheniramine at 1 to 2 mg, orally, every 12 hours may lessen clinical signs of disease in some cats. Several newer antihistamines are now available (see Table 30-1), and because response to therapy is variable from patient to patient, an alternative drug should be tried if no improvement is seen with the initial choice. Moistening therapies such as nebulization and saline nasal drops can help loosen secretions and soothe mucosa, particularly in drier climates.
Neoplasia
Neoplasia of the nasal cavity is relatively rare in the cat compared with the dog; lymphoma is the most common tumor type, followed by adenocarcinoma and squamous cell carcinoma.12,33,65 Lymphoma is treated with chemotherapy, often in conjunction with radiation therapy, and has potential for a good long-term prognosis.89 Palliative radiation therapy is indicated for other nasal neoplasms, and surgical debulking is generally not required.62 Prognosis depends on the aggressiveness and extent of the tumor, which is best determined by CT scan. Piroxicam administered at 0.3 mg/kg, orally, every 48 to 72 hours can control inflammation and clinical signs of disease in some cats with nonlymphoproliferative nasal neoplasia. Meloxicam (0.1 mg/kg, orally, every other day) may be another efficacious alternative. However, neither drug may result in antitumor effects against squamous cell carcinoma because it has been shown that there is minimal expression of cyclooxygenase-2 in this cancer type in the cat.15 If nonsteroidal antiinflammatory therapy is used, the cat should be monitored for renal and gastrointestinal side effects (e.g., PCV to assess for gastrointestinal hemorrhage and renal values) because these are potential side effects of this drug family.
Spontaneous Disorders
Trauma
Trauma to the nasal cavity most commonly results in massive hemorrhage, and thus initial evaluation should include assessment and treatment for hypovolemic shock. Nasal tissue may be easily damaged because of its fragile structure. Placement of a nasal cannula may aid in airflow and allowing healing that maintains a nasal passage. It may take 2 to 3 weeks for healing to occur. Generally, surgical correction of fractures in the nasal cavity is not necessary, although solitary bone fragments should be removed to prevent the formation of sequestra. Trauma may also lead to chronic complications as a result of damage to the nasal passage.33
Foreign Bodies
Nasal foreign bodies are more common in cats than generally realized.33,82 In dogs the foreign material is usually inspired into the anterior nares and is found in the ventral meatus just caudal to the nares. Most nasal foreign bodies in cats are plant material that lodges above the soft palate after coughing or vomiting. Clinical signs may include sneezing, reverse sneezing, gagging, and repeated attempts at swallowing. Retroflex rhinoscopic examination of the nasopharynx can sometimes confirm diagnosis and aid in removal of the object. Nasal lavage under general anesthesia is often more effective. The cuff of the endotracheal tube should be checked for full inflation before performing nasal lavage with saline administered under pressure. In cats lavaging from the anterior nares caudally is recommended. Gauze should be placed in the oropharyngeal area, and then a 20-, 35-, or 60-mL syringe can be used to forcefully flush saline through the nose while the nares are being pinched off to create pressure. Material flushed from the nose (or oropharynx) should be caught on the gauze and examined for foreign material.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree