Chapter 26 Reproductive Toxicology of the Male Companion Animal
IS REPRODUCTIVE TOXICOLOGY OF THE MALE CLINICALLY IMPORTANT?
Historically, there have been few data on the effects of toxicological and pharmacological compounds on the actual fertility of the male animal. This dearth of information is particularly true for the companion animal male even though subfertility in a valuable stud animal can have economically and genetically devastating effects on a breed or familial line.1,2
Recently the critical role of the male gamete (sperm) in predicting the final reproductive outcome has become a matter of interest.3–5 Specifically, it is now recognized that toxins that affect the reproductive system need not cause total sterility of the male to have a deleterious effect on a population.6–8 Rather, compounds that damage sperm function, including the deoxyribonucleic acid (DNA) (chromatin), can lead to fertilization failure, embryo loss, spontaneous abortion of the fetus, and birth defects or even disease in the offspring (e.g., cancer).
In addition, studies of wildlife and human populations suggest that sperm counts throughout the world may be declining, with a concomitant increase in the incidence of reproductive tract pathological conditions.9,10 These findings have greatly intensified research into male reproductive disorders and the impact on them of external factors, such as environmental conditions, toxins, and pharmaceuticals. This is particularly true in light of the fact that many compounds that negatively affect male fertility are stored in the animal over years (bioaccumulation) and can function synergistically with each other.10,11
This chapter outlines the pathophysiology by which male fertility can be damaged after toxicant exposure. Specific pharmacological and chemical compounds that can affect the reproductive outcome of the male are then reviewed. Because of the very limited availability of scientific data specific to toxicology of the intact male dog or cat, reproductive toxins in other species (preponderantly human or rodent) are included in this chapter.
HOW DO TOXINS AFFECT MALE REPRODUCTION?
Pathophysiology of toxin exposure in the male
Fertility of mammalian males, including the dog and cat, can be altered by disrupting the endocrine system or sperm production. Disruption of the endocrine system can affect sexual differentiation of the fetus, sexual maturation at puberty, libido or male behavior in the adult animal (e.g., delivery of sperm to the female), and actual sperm production.11–13 Abnormalities of sperm production (including numbers of sperm and sperm shape and motility) and damage to sperm chromatin (DNA) before fertilization can occur by separate mechanisms and are not necessarily correlated. Both poor sperm quality and abnormal sperm DNA can deleteriously affect the reproductive outcome.5,7,14 Recent studies into the mechanisms of sperm DNA damage have shown that there are several types of sperm abnormalies that can occur even in the face of normal fertilization (i.e., sperm quality is sufficient to support fertilization); however, abnormal DNA is passed on to the offspring, resulting in fetal losses and potential pathological conditions.4–715
Normal and abnormal endocrine function of the male
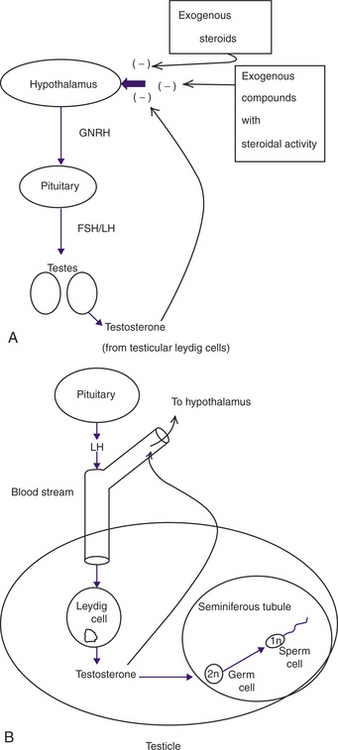
Hormonal control of the mammalian male involves a continuous feedback loop between the hypothalamus, the pituitary, and the gonads (testicles) as depicted in Figure 26-1, A.12,16,17 This hypothalamic-pituitary-gonadal (HPG) axis controls testosterone secretion from the Leydig cells in the testicle (Figure 26-1, B). Adequate testosterone levels in the testicle are requisite for normal sperm production. The level of testosterone in the bloodstream affects libido and secondary sex characteristics and controls the hypothalamic secretion of gonadotropin-releasing hormone, which leads to the production of testicular testosterone.13 Blood levels of testosterone are two to three times lower than the levels of testicular testosterone required for sperm production. Therefore you can never give an animal enough systemic testosterone (or other anabolic steroid) to improve sperm production.18 Rather, any exogenous steroid or compound with steroidal activity given to an animal will interfere with the HPG axis, causing a decrease in hypothalamic activity and less testosterone in the testicle, even though libido and other peripheral sex characteristics may be improved.
Many toxicants act by interfering with the HPG axis and altering hormone production, including testosterone levels in the testicle, which in turn disrupts sperm production.19 The level of exposure to exogenous steroids or compounds with steroid-like activity required to interfere with fertility is highly variable among individuals. Some of this variability may be due to individual animal responses after exposure to compounds with steroidogenic activity. However, breed and species differences may exist as well. For example, metabolism of phytoestrogens (from dietary soybean products) may be less efficient in cats than in other species, possibly leading to infertility in some animals.20 Also, in humans, there are ethnic and racial differences in the sensitivity of the individual to exogenous steroids and their subsequent effect on spermatogenesis.21
Normal and abnormal sperm production
The production of sperm (spermatogenesis) involves a complex process of cellular divisions leading from germ cells (the “parent” cells in the tubules of the testicles) to functionally mature sperm cells that can fertilize an egg (Figure 26-1, B). This process allows the germ cells containing both chromosomes from the father (2n) to rapidly divide, providing a continual source of sperm with one set of chromosomes (1n) for ejaculation and the production of offspring.18
The high rate of division of testicular germ cells makes them uniquely sensitive to toxic agents.11,22 If the germ cells are damaged, several possible outcomes can occur. Sperm production may be decreased or cease; abnormal sperm (e.g., in shape or motility) can be produced, or the chromosomal material (DNA) of the sperm can become abnormal.14 Whether or not these changes are reversible depends on whether or not the insult was sufficient to cause permanent damage to the parent germ cell in the testicle. There is a great deal of individual variation in the intensity of exposure necessary to affect the germ cells in the testicle irreversibly. The more primitive the germ cell affected (in the continuum of spermatogenesis) by an exposure, the more long-lasting and potentially detrimental are the results on future reproduction. In general the process of spermatogenesis (from germ cell to mature sperm cell) takes about 60 days.18 Therefore, to determine whether observed abnormalities in sperm production are permanent or temporary requires clinical observation through several 60-day periods.23
Abnormal sperm production can occur following cellular insult to the germ cell, leading to disruption in spermatogenesis; decreases in testicular testosterone, which disrupts spermatogenesis; or damage to the DNA of germ cell or sperm chromosomes at any stage of development. With several toxins, damage to sperm DNA can occur before any observable loss of sperm quality or fertilization is seen.7,15,24 Such sperm can lead to abnormal reproductive outcomes, such as early embryonic death, spontaneous abortion, and possible abnormality in any resulting offspring.
Potential problems observed in male fertility disorders following toxin exposure
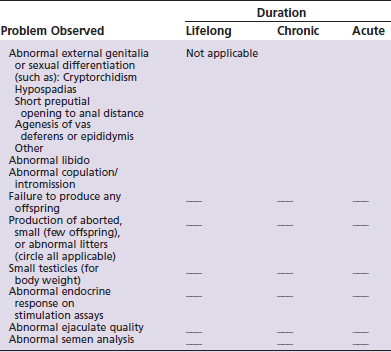
The checklist shown in Table 26-1 can be used to identify potential male reproductive disorders that should include toxin exposure as part of the differential diagnosis.25 Diagnostic and therapeutic protocols for subsequent work-up of the companion animal are briefly outlined in Table 26-2.26–28 For each problem identified, one must determine (as appropriate) whether the duration has been lifelong, chronic (6 months or more), or acute (less than 6 months). It is important to note that by the time a clinical problem in reproduction is observed, acute (one time) exposures to a toxin may have occurred weeks or months in the past. Therefore recollection of past possible exposures is warranted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree