Chapter 25 Reproductive Toxicology of the Female Companion Animal
FEMALE REPRODUCTIVE TOXICOLOGY
Because the features of reproduction differ from other areas of health function, an alternative approach for reproductive risk assessment is required. For example, reproduction is only intermittently expressed in contrast to the continuous function of other organ systems required for survival. Furthermore, although other types of toxicity can be followed directly by assessment of exposure and function in a single animal, reproductive function can only be fully evaluated in a mating pair and their offspring, and therefore requires attempts at pregnancy for complete evaluation.1 Resources and approaches are identified for the assessment of risk for reproductive toxicity.
TARGET SITES FOR THE EFFECTS OF REPRODUCTIVE TOXICANTS IN THE FEMALE SYSTEM
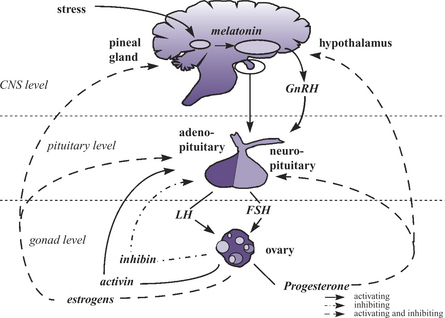
The following section reviews some sites along the hypothalamic-pituitary-ovarian-uterine-axis (HPOUA) (Figure 25-1) that are vulnerable to disruption by toxicant injury. The HPOUA is regulated in a complex manner that requires the appropriate interaction of the central nervous system (CNS) with the ovaries and reproductive tract. To achieve the successful outcome of live, normal, healthy offspring, the individual components of the HPOUA must each function properly, and this involves critical timing and integration of signaling among the sites involved. The overall regulation originates in the CNS with its output of neuroendocrine signals from the hypothalamus and pituitary. The ovary is the site of direct regulation of cyclicity and reproductive function, and the reproductive tract is the site where the gametes join and the embryo or the fetal unit develops and grows, and is then delivered to the external environment. The mammary glands, which are under the regulation of the neuroendocrine system, are also essential to the early survival of the offspring.
Hypothalamus
The hypothalamus has permissive control of the estrus cyclicity of the female through the pulsatile release of gonadotropin-releasing hormone (Gn-RH). The timing, frequency, and amount of Gn-RH released are essential to critical events in the female reproductive cycle, including the initiation of estrus and ovulation. The hypothalamus is modulated by both positive and negative feedback from factors released by the CNS, ovaries, and uterus (e.g., neurotransmitters, androgens, estrogens, and progestogens) (Figure 25-1). The release of Gn-RH into the hypophyseal portal system facilitates the interaction of Gn-RH with the anterior pituitary to initiate synthesis, storage, and secretion of the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Disruption of the normal pattern of Gn-RH release can result in a decrease in female fecundity and fertility. Altered frequency or amplitude of the Gn-RH pulse could be the result of a disruption in the stimulatory or inhibitory pathways that regulate Gn-RH release. Catecholamines, dopamine, serotonin, γ-aminobutyric acid (GABA), and endorphins all have the potential to alter the release of Gn-RH.1 Therefore xenobiotics that are agonist or antagonists of these compounds have the potential ability to modify Gn-RH release, thereby interfering with pituitary function.
Drugs that are commonly used, such as sodium pentobarbital and atropine, have been shown to block surges in Gn-RH intended to initiate the pathway for ovulation in rats.2 Exposure to xenobiotics during the proestrus period has the greatest potential to delay or block the Gn-RH release necessary for ovulation. CNS-active drugs, such as opiates and delta-9-tetrahydrocannabinol, the major psychoactive component of marijuana, have been shown to disrupt the hypothalamic-pituitary axis.3–6 The effects in the female are disruption of estrus cyclicity, delayed sexual development, and dysfunction of the ovary. Thiram (tetramethylthiuram disulfide), a fungicide that inhibits dopamine synthesis, either blocks ovulation or causes a decrease in embryo survival when exposure occurs during proestrus in rodents.7,8 Both methanol and sodium valproate (valproic acid) have been shown to disrupt the hypothalamic-pituitary axis and block ovulation.9,10 The mechanism by which methanol works is unknown. However, valproic acid works by disrupting the endocrine system by acting as a GABA receptor agonist. A number of pesticides have the endocrine-like activity that leads to disruption of the hypothalamic-pituitary axis. Atrazine fed to rats increases the rate of pseudopregnancy.11,12 Lindane has been shown to delay the onset of puberty in the rat by its interactions with the GABA receptor.13
Assessment of Gn-RH release is impractical in companion animal medicine. However, procedures for the assessment of pituitary function as the target organ for Gn-RH release are available.14 Gn-RH agonists are available commercially, and their misuse can also lead to reproductive disorders.15
Anterior pituitary
The anterior pituitary secretes three hormones (FSH, LH, and prolactin) that are essential for female fecundity. Their critical roles are maintaining ovarian cyclicity, governing follicular recruitment and maturation, ovulation, and luteinization. The anterior pituitary is also a site of positive and negative feedback from the ovary (Figure 25-1). The appropriate pattern of release of FSH and LH during the estrus cycle controls the events of normal follicular development, whereas an alteration in the secretion pattern may result in abnormal follicular development, acyclicity, and ovarian atrophy.
Toxicant-induced alterations in the synthesis, storage, or secretion of gonadotropins can seriously disrupt female reproductive function. Steroid agonists and antagonists may initiate an inappropriate release of gonadotropins from the pituitary, thereby disrupting the ovarian cycle. Xenobiotics can potentially interfere with normal feedback dynamics of ovarian steroids. For example, diethylstilbestrol and the pesticide methoxychlor both have estrogenic properties and have been shown to alter normal pituitary function in rodents.5 Bromocriptine, acting as a dopamine agonist, suppresses prolactin release by the pituitary, and haloperidol, a dopaminergic antagonist, increases prolactin levels.16 Chemicals that alter endocrine homeostasis may induce the secretion of steroid-metabolizing enzymes, thus reducing steroid half-life and the circulating level of steroids at the pituitary level. Exposure to metal cations, such as cadmium, cobalt, nickel, zinc, or lead, may produce alterations in pituitary function.16 Protocols for the assessment of pituitary function are available.14 However, in the assessment of the bitch and queen, one must always take into consideration the basic reproductive patterns of each species, including seasonality.
Ovary
The basic functional unit of the ovary, the follicle, maintains the delicate hormonal environment necessary to support the growth and maturation of an oocyte. The pattern of follicular development depends on processes involving both intraovarian and extraovarian regulation. As the follicle develops from a primordial to a graafian follicle, numerous morphological and biochemical changes occur in concert with endocrine stimulation and feedback. These characteristics suggest a number of potential sites for xenobiotic interaction. Each developmental step of follicular maturation has a varying degree of sensitivity depending on the toxicant. The degree of impaired fecundity is dependent on the follicle type affected. A toxicant that impairs the development of primordial follicles is not immediately identifiable; rather, the effects (i.e., a shortened reproductive lifespan) would not be noticed for several years. On the other hand, toxicity to the antral or preovulatory follicles would result in a reduction in litter size.
Oocytes are vulnerable to damage or destruction by xenobiotics. Potential sites of injury include the processes integral to oocyte maturation and meiotic cell division. Alkylating agents have been shown to destroy oocytes.17 Lead has also been observed to produce ovarian toxicity characterized by follicular atresia in rodents and nonhuman primates.18,19 Other metals, including mercury and cadmium, have also been shown to produce ovarian damage that may be mediated through oocyte toxicity.1
The ovarian toxicity of polycyclic aromatic hydrocarbons (PAHs) and benzo[a]pyrene (BP) has been studied extensively in laboratory animals.20–25 BP acts as an indirect toxicant in the induction of ovarian toxicity. For BP to cause ovarian toxicity, it first must be metabolized to an active metabolite by cytochrome P450 enzymes. This example is extremely important as one investigates the types of chemical exposure an animal receives and the differences in metabolism among species. For example, one only needs to remember the differences in metabolism of acetaminophen between dogs and cats. In addition, a female dog or cat exposed to cigarette smoke may receive sufficient exposure to PAHs to alter the normal reproductive processes.26
ASSESSMENT OF REPRODUCTIVE DISORDERS
It is beyond the scope of this chapter to thoroughly review the processes and methods used to assess a reproductive disorder. There are several reference articles on this topic.9,27–30 Briefly, the initial challenge faced by the clinician is the accurate determination that a reproductive disorder is in fact present. The first step is to define the problem precisely by ascertainment of the necessary information, including a thorough history, physical examination, and laboratory evaluation. The next step is to establish a cause of the defined problem that will allow an assessment of the reversibility of the problem. However, determination of the cause of the reproductive disorder may not identify the initial causative agent, but rather an effect of the toxicant.
Depending on the nature of the disorder, treatment is available for a limited number of conditions. The treatment of reproductive disorders is beyond the scope of this chapter and has been reviewed elsewhere.15
ASSESSMENT OF EXPOSURE
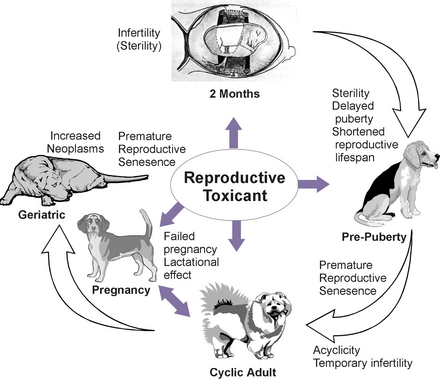
The length of exposure is critical information. The possible effects of chronic exposure to low levels of reproductive toxicants are more difficult to determine because of the potential for additive or cumulative effects (Figure 25-2). In addition, fertility problems produced by xenobiotic exposure may go unrecognized until the next generation reaches reproductive maturity. For example, in utero exposure to a toxicant that disrupts the development of the internal reproductive organs would be unnoticed until the female offspring reach the age of reproductive activity. In this example, the ability to gather information about the level and nature of exposure to a reproductive toxicant is extremely difficult.
Pregnancy and lactation
As a general rule, administration of drugs is avoided during pregnancy and lactation. However, occasionally the benefits of drug administration during these periods outweigh the risks. The use of drugs during pregnancy has been thoroughly reviewed (Table 25-1). In addition, accidental exposures to drugs and environmental chemicals do occur during pregnancy and lactation. To determine the risks associated with such exposure, a number of factors must be established: the gestational period that exists during the exposure and the length, level, and route of the exposure. Potential outcomes of inadvertent exposures include teratogenic congenital malformations or reduced litter size or abortion. The available database on the effects of xenobiotic exposure during pregnancy and lactation is extremely limited for dogs and cats. Case reports documenting the outcomes of pregnancies following exposure to drugs or environmental chemicals are extremely valuable resources.
Table 25-1 Safety of Drugs in Pregnancy
| Drug | Recommendation | Comments |
|---|---|---|
| Antimicrobial Drugs | ||
| Amikacin | C | Aminoglycoside antibiotics easily cross the placenta and may cause 8th CN toxicity or nephrotoxicity |
| Amoxicillin | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Ampicillin | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Carbenicillin | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Cephalosporins | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Chloramphenicol | C | May decrease protein synthesis in fetus, particularly in bone marrow |
| Ciprofloxacin | D | Do not use during pregnancy; quinolones have been associated with articular cartilage defects |
| Clavulanic acid-amoxicillin (Clavamox, Beecham) | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Clindamycin | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Cloxacillin | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Dicloxacillin | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Doxycycline | D | Tetracyclines can cause bone and teeth malformations in fetus and may cause toxicity in mother |
| Enrofloxacin | D | See ciprofloxacin |
| Erythromycin | A | Appears to be safe except for erythromycin estolate, which has been shown to increase the risk of hepatotoxicity in women |
| Drug | Recommendation | Comments |
|---|---|---|
| Gentamicin | C | Aminoglycoside antibiotics easily cross the placenta and may cause 8th CN toxicity or nephrotoxicity; however, specific toxicities from gentamicin have not been reported, and it may be used for a serious infection in place of a suitable alternative |
| Hetacillin | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Kanamycin | C | Aminoglycoside antibiotics easily cross the placenta and may cause 8th CN toxicity or nephrotoxicity |
| Lincomycin | A | Crosses the placenta but has not been shown to cause problems in fetus |
| Metronidazole | C | Teratogenic in laboratory animals, but there is no information for dogs and cats. It should be avoided during the first three weeks of pregnancy |
| Neomycin | A | Not absorbed sufficiently to cause systemic effects after oral administration |
| Oxacillin | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Oxytetracycline | D | Toxic to fetus and may increase risk of hepatitis in mother (see tetracycline) |
| Penicillin G (benzyl penicillin) | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Streptomycin | D | See gentamicin. Streptomycin is associated with higher incidence of 8th CN toxicity than other aminoglycosides |
| Drug | Recommendation | Comments |
|---|---|---|
| Sulfonamides | B | Sulfonamides cross the placenta and have produced congenital malformations in rats and mice, but problems have not been reported in dogs or cats; in people, they have caused neonatal icterus when administered near term; avoid long-acting sulfonamides |
| Tetracycline | D | Tetracyclines can cause bone and teeth malformations in fetus and may cause toxicity in mother |
| Trimethoprim-sulfadiazine (Tribrissen, Coopers) | B | Manufacturer states that it is safe during pregnancy in dogs; see also trimethoprim and sulfonamides |
| Trimethoprim | B | Teratogenic in rats but probably safe in other species; folate antagonism and bone marrow depression are possible with prolonged use |
| Ticarcillin | A | Crosses the placenta but has not been shown to be harmful to fetus |
| Tobramycin | C | Aminoglycoside antibiotics easily cross the placenta and may cause 8th CN toxicity or nephrotoxicity |
| Tylosin | B | No information is available |
| Antifungal Drugs | ||
| Amphotericin-B | C | There are no known teratogenic effects, but amphotericin is extremely toxic; use only if the disease is life threatening, in absence of a suitable alternative |
| Drug | Recommendation | Comments |
|---|---|---|
| Griseofulvin | D | Teratogenic in rats; causes multiple skeletal and brain malformations in cats |
| Ketoconazole | B | Teratogenic and embryotoxic in rats; antiandrogenic; stillbirths have been reported in dogs |
| Miconazole | A | Apparently safe if applied topically |
| Antiparasitic Drugs | ||
| Amitraz | C | Manufacturer states that reproduction studies have not been done; no information available |
| Bunamidine | A | Has been administered to pregnant bitches without problems and is safe in pregnant cats; slight interference with spermatogenesis has been seen in male dogs |
| Diethylcarbamazine | A | Manufacturer states that the drug may be given to dogs throughout gestation |
| Dithiazanine iodide (Dizan, TechAmerica) | B | No information is available; iodide salts may cause congenital goiter if administered for prolonged periods during pregnancy |
| Fenbendazole | A | Safe; has been administered to pregnant bitches without producing adverse effects |
| Dichlorvos (Task, Solvay) | B | Caution is advised when administering cholinesterase inhibitors to pregnant animals; it should not be administered to puppies or kittens, but studies in pregnant dogs and cats suggest that there are no adverse effects during pregnancy |
| Drug | Recommendation | Comments |
|---|---|---|
| Ivermectin | A | Safe; reproduction studies in dogs, cattle, horses, and pigs have not shown adverse effects |
| Levamisole | C | No information available |
| Mebendazole | A | Safe in reproduction studies in dogs; it was not teratogenic or embryotoxic |
| Piperazine | A | Safe; no known contraindications for the use of piperazine |
| Praziquantel | A | Safe; no adverse effects were seen when tested in pregnant dogs and cats |
| Pyrantel | A | Safe; toxicity studies have not shown any adverse effects |
| Thenium | A | Safe; manufacturer states that except in young puppies, there are no known contraindications |
| Thiabendazole | B | Thiabendazole is not teratogenic in laboratory animals, but high doses have produced toxemia in ewes |
| Thiacetarsamide (Caparsolate sodium, CEVA) | C | No specific information regarding toxicity to fetus is available; can be hepatotoxic and nephrotoxic, and heartworm adulticide should be postponed until after parturition |
| Trichlorfon | C | Caution is advised when administering organophosphates to pregnant animals; congenital toxicoses have been reported following administration to pregnant sows; manufacturer states that trichlorfon should not be administered to pregnant mares, but there are no recommendations for dogs and cats |
| Drug | Recommendation | Comments |
|---|---|---|
| Anticancer Drugs | ||
| Azathioprine | C | May produce congenital malformations but has been used in pregnant women safely; may be a suitable alternative to other drugs when immunosuppressive therapy is required |
| Doxorubicin hydrochloride (Adriamycin, Adria) | C | May produce malformations in newborn or embryotoxicity |
| Chlorambucil | C | May produce malformations in newborn or embryotoxicity |
| Cisplatin | C | May produce congenital malformations, embryotoxicity, or nephrotoxicity |
| Cyclophosphamide | C | May produce malformations in newborn or embryotoxicity |
| Methotrexate | C | May produce malformations in newborn or embryotoxicity |
| Vincristine | C | May produce malformations in newborn or embryotoxicity |
| Analgesic Drugs | ||
| Acetaminophen | C | Safety not established in dogs; toxic in cats |
| Aspirin | C | Embryotoxicity has been seen in laboratory animals but not in other species; late in pregnancy, may produce pulmonary hypertension and bleeding problems |
| Flunixin meglumine | C | Safety in pregnancy has not been determined |
| Gold (aurothioglucose) | D | Laboratory animal studies clearly show increased congenital malformations |
| Drug | Recommendation | Comments |
|---|---|---|
| Ibuprofen | C | Safety in dogs and cats not established |
| Indomethacin | C | Can be toxic in adult dogs; can cause premature closure of ductus arteriosus if administered near term |
| Phenylbutazone | C | Safety has not been established. Long-term use can depress bone marrow |
| Salicylates | C | Embryotoxicity has been seen in laboratory animals but not in other species; late in pregnancy, may produce pulmonary hypertension and bleeding disorders |
| Anesthetic and Preanesthetic Drugs | ||
| Acepromazine | B | Phenothiazines should be avoided near term; they may produce neonatal CNS depression |
| Atropine | B | Crosses the placenta and has been used safely but may cause fetal tachycardia |
| Butorphanol | B | Safe for short-term use; neonatal depression can be treated with naloxone |
| Codeine | B | Safe for short-term use; neonatal depression can be treated with naloxone |
| Diazepam | C | See anticonvulsants |
| Fentanyl | B | Safe for short-term use; neonatal depression can be treated with naloxone |
| Glycopyrrolate | B | Safe; does not cross placenta as readily as atropine. Studies in rats and rabbits have not revealed teratogenic effects |
| Drug | Recommendation | Comments |
|---|---|---|
| Halothane | C | Decreased learning ability has been reported in rats after in utero exposure; depression may be seen in neonates after cesarean section; excessive uterine bleeding may be seen when administered during cesarean section |
| Isoflurane | B | Probably safe; depression may be seen in neonates after cesarean section |
| Ketamine | B | Probably safe; depression may be seen in puppies delivered by cesarean section; may increase intrauterine pressure and induce premature labor |
| Lidocaine | A | All local anesthetics appear to be safe when used for a local nerve block or epidural anesthesia |
| Meperidine | B | Opiates can produce neonatal sedation and respiratory depression, but the effects can be reversed with the administration of naloxone |
| Methoxyflurane | C | Neonatal depression is seen when used for cesarean section |
| Morphine | B | Opiates can produce neonatal sedation and respiratory depression, but the effects can be reversed with the administration of naloxone |
| Naloxone | A | Has been shown to be safe when administered to newborns within a few minutes after birth |
| Nitrous oxide | B | Probably safe; used frequently for cesarean section without adverse effects |
| Drug | Recommendation | Comments |
|---|---|---|
| Oxymorphone | B | Opiates can produce neonatal sedation and respiratory depression, but the effects can be reversed with the administration of naloxone |
| Pentobarbital | D | Associated with high incidence of neonatal mortality |
| Thiamylal | C | Easily crosses the placenta; all barbiturates produce respiratory depression in fetus; however, thiobarbiturates are not as toxic as pentobarbital |
| Thiopental | C | Easily crosses the placenta; all barbiturates produce respiratory depression in fetus; however, thiobarbiturates are not as toxic as pentobarbital |
| Gastrointestinal Drugs | ||
| Antacids | A | Safe; not absorbed systemically |
| Antiemetics | B | Probably safe if administered short term |
| Cimetidine | B | Safety has not been established, but no reports of toxicity in humans |
| Dimenhydrinate | B | Safe if used short term |
| Diphenhydramine | B | Safe if used short term |
| Diphenoxylate | C | Studies have reported adverse effects in laboratory animals, but no adverse effects have been reported in pregnant dogs, cats, and humans |
| Laxatives | B | All laxatives, except castor oil (Squibb), are considered safe if they are used short term; castor oil causes premature uterine contractions |
| Loperamide | C | Studies have reported adverse effects in laboratory animals, but no adverse effects have been reported in pregnant dogs, cats, and humans |
| Drug | Recommendation | Comments |
|---|---|---|
| Methscopolamine | C | Safety not established |
| Metoclopramide | B | Safe in laboratory animals, but no studies available for cats or dogs |
| Misoprostol | D | Synthetic prostaglandin, causes a termination of pregnancy |
| Prochlorperazine | B | No reports of toxicity when administered short term |
| Ranitidine | B | Safety has not been established, but no reports of toxicity were reported in humans |
| Sucralfate | A | Probably safe. Not absorbed systemically |
| Sulfasalazine | B | Salicylate component is not absorbed enough to produce adverse effects; sulfonamide may produce neonatal icterus when used near term (see text) |
| Cardiovascular Drugs | ||
| Atropine | B | Probably safe but may produce fetal tachycardia |
| Captopril | C | Has been shown to be embryotoxic in laboratory animals and goats |
| Digitalis | A | Probably safe; no adverse effects seen in humans and laboratory animals |
| Furosemide | B | No adverse effects have been reported |
| Dopamine | B | Probably safe at therapeutic doses |
| Heparin | B | Does not appear to cross placenta |
| Hydralazine | B | Probably safe; have been reports of minor toxicity in rats, but it has been administered safely to pregnant women |
| Isoproterenol | C | May cause fetal tachycardia; beta-adrenergic drugs inhibit uterine contractions |
| Drug | Recommendation | Comments |
|---|---|---|
| Lidocaine | B | Probably safe; may cause fetal bradycardia |
| Nitroglycerin | C | No information available |
| Nitroprusside | C | There is a risk of fetal cyanide toxicity with prolonged use |
| Procainamide | B | Probably safe; may cause fetal bradycardia |
| Propranolol | C | May cause fetal bradycardia, respiratory depression, and neonatal hypoglycemia; avoid use near term |
| Quinidine | B | Probably safe; may cause fetal bradycardia |
| Theophylline | B | No reports of adverse effects |
| Thiazide diuretics | C | May cause increased incidence of perinatal mortality |
| Warfarin | D | Causes embryotoxicity and congenital malformations; neural tube defects in laboratory animals and humans |
| Anticonvulsant Drugs | ||
| Diazepam | C | Has been associated with congenital defects in mice, rats, and people |
| Phenobarbital | B | Has been associated with rare congenital defects and bleeding tendencies in newborn but may be safer than other anticonvulsants |
| Phenytoin | C | Teratogenic in rats, mice, and people |
| Primidone | C | Same risks as phenobarbital and has been associated with increased incidence of hepatitis in adult dogs |
| Valproic acid | C | May cause congenital malformations; causes neural tube malformations in humans |
| Drug | Recommendation | Comments |
|---|---|---|
| Muscle Relaxants | ||
| Dantrolene | C | Safety not established |
| Dimethyltubocurarine | B | Quarternary base with negligible placental transfer; does not affect the fetus unless administered in large doses |
| Gallamine | B | Quarternary base with negligible placental transfer; does not affect the fetus unless administered in large doses |
| Methocarbamol | C | Safety not established; manufacturer states that it should not be administered during pregnancy |
| Pancuronium | B | Quarternary base with negligible placental transfer; it does not affect the fetus unless administered in large doses |
| Succinylcholine | B | Quarternary base with negligible placental transfer; does not affect the fetus unless administered in large doses |
| Endocrine Drugs | ||
| Betamethasone | C | Corticosteroids have been associated with increased incidence of cleft palate and other congenital malformations, and they may induce premature labor and abortion in dogs |
| Cortisone | C | Corticosteroids have been associated with increased incidence of cleft palate and other congenital malformations, and they may induce premature labor and abortion in dogs |
| Drug | Recommendation | Comments |
|---|---|---|
| Dexamethasone | C | Corticosteroids have been associated with increased incidence of cleft palate and other congenital malformations, and they may induce premature labor; dexamethasone has caused abortion and fetal death in dogs |
| Diethylstilbestrol (DES) | D | Malformation of male and female genitourinary systems |
| Estradiolcypionate (ECP) | D | Malformation of male and female genital tracts and bone marrow depression |
| Flumethasone | C | Corticosteroids have been associated with increased incidence of cleft palate and other congenital malformations, and they may induce premature labor and abortion in dogs |
| Mitotane (o, p-DDD) | D | Adrenocortical necrosis |
| Prednisolone | C | Although prednisolone has been administered to pregnant women without adverse effects, caution is advised (see dexamethasone). Prednisolone may be used in serious diseases in absence of a suitable alternative |
| Stanozolol | D | Manufacturer states that it should not be administered to pregnant dogs and cats |
| Testosterone | D | Causes masculinization of female fetus |
| Thyroxine | B | Does not cross placenta easily and has not been associated with any problems |
| Drug | Recommendation | Comments |
|---|---|---|
| Miscellaneous Drugs | ||
| Ammonium chloride | B | May cause fetal acidosis; discontinue use during pregnancy |
| Aspartame (Nutra-Sweet) | A | No risk |
| Dimethylsulfoxide (DMSO) | C | Teratogenic in laboratory animals; manufacturers state that it should not be applied to breeding animals |
A: Probably safe. Although specific studies may not have proved the safety of all drugs in dogs and cats, there are no reports of adverse effects in laboratory animals or in women.
B: Safe for use if used cautiously. Studies in laboratory animals may have uncovered some risk, but these drugs appear to be safe in dogs and cats or these drugs are safe if they are not administered when the animal is near term.
C: These drugs may have potential risks. Studies in people or laboratory animals have uncovered risks, and these drugs should be used cautiously, as a last resort when the benefit of therapy clearly outweighs the risks.
D: Contraindicated. These drugs have been shown to cause congenital malformations or embryotoxicity.
From Papich MG: Effects of drugs on pregnancy. In Kirk RW (ed): Current Veterinary Therapy X: Small Animal Practice. Philadelphia, WB Saunders, 1989.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree