Chapter 9 Reproductive Diseases
The scope of theriogenology is beyond simple summation within a single chapter of a general textbook. Therefore no effort will be made to cover all gynecologic and reproductive topics. Emphasis in this chapter will be directed toward diseases of the reproductive tract that cause signs of illness or disease and require medical attention in dairy cattle. Standard theriogenology textbooks should be consulted for more in-depth reading and discussions of infertility, endocrinology, dystocia, and abortion.
DISEASES OF THE UTERUS
Hydrops
Signs
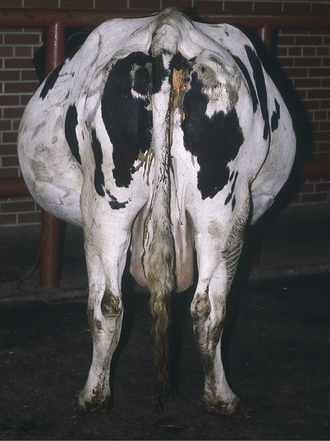
The major outward sign of hydrops is progressive abdominal distention during the last trimester that worsens to such a degree as to decrease appetite and cause difficulty in moving or rising (Figure 9-1). Although abnormal fetuses causing hydramnios rarely can cause abdominal distention as early as the midtrimester, this is much less common than hydrops that appears during the last 4 to 6 weeks of pregnancy. The distended uterus takes up so much room in the abdomen that affected cows have reduced appetites because of visceral compression. Weakness results both from reduced feed consumption and from the increased weight of uterine fluids. Secondary ketosis and other metabolic conditions are possible complications as a result of decreased feed intake and fetal nutritional needs—especially if twins are present in cows with hydrallantois. Rectal examination may help to differentiate hydramnios and hydrallantois. In hydramnios, the fetus and placentomes are palpable, but the uterine horns are more difficult to palpate. In hydrallantois, the distended uterine horns appear to fill the abdomen, but palpation of the fetus and placentomes may not be possible because the uterus is stretched tightly by the increased fluid content. Rectal or transabdominal ultrasound examination is helpful in making a diagnosis. Unless the conditions are diagnosed promptly, musculoskeletal complications such as exertional myopathy, hip injuries, hip luxations, and femoral fractures can occur because of struggling to rise or slipping brought on by the tremendous abdominal weight. (As much as 200 liters, or 440 pounds, of additional fluid may accumulate in the case of hydrops allantois.) Rupture of the prepubic tendon and ventral hernias also may occur. Because hydrallantois tends to cause more rapid fluid accumulation, musculoskeletal injuries appear to be more common with this condition than in the slowly enlarging hydramnios patients.
Treatment
Dr. Rebhun’s preference was to induce parturition with IM injections of 30 to 40 mg prostaglandin F2α (PGF2α) and 20 mg of dexamethasone. Cervical relaxation may also be attempted by the manual application of synthetic prostaglandin E in the external os and cervical canal. Specific human gynecologic preparations of prostaglandin E are very expensive, but I have had success using misoprostol (1000 mg mixed in sterile obstetrical gel applied thrice daily) as an aid to cervical ripening in some cattle. Pregnant women should not handle this product! Most cattle treated with this combination calve within 24 to 48 hours and should be monitored closely. IV fluids—hypertonic saline is especially useful—are administered to cattle showing any degree of dehydration or inappetence. Supportive glucose or calcium may be indicated when ketosis or hypocalcemia is present. Treated cows are kept in well-bedded box stalls with good footing and are monitored closely because they may require assistance in calving. Milking is started as soon as the fetus is delivered, even when little milk is present in the udder. Most fetuses or calves delivered are abnormal, small, or nonviable. If the calf is more valuable than the cow and ultrasound examination indicates a viable fetus, treatment of the cow with dexamethasone and cesarean section 24 hours later would provide the best chance of having a live calf. In the case of an apparently viable calf, colostrum should be provided from another cow because the patient seldom has normal colostrum. Prognosis is better for those cows near term. Metritis and retained fetal membranes (RFM) should be anticipated and prophylactic antibiotic therapy instituted. Clinicians should anticipate that the cow will have a large, potentially enormous, atonic uterus after calving and will experience highly protracted and challenging involution. Although fetal kidney anomalies are more likely to be associated with hydrallantois, Dr. Rebhun once treated a hydramnios cow that subsequently delivered a calf having polycystic kidneys. Cattle with hydrallantois that survive delivery and avoid life-threatening postpartum metritis should not be bred again. If fetal hydrops causes dystocia, the excessive fluid can be drained to allow fetal extraction.
Rupture of the Prepubic Tendon and Ventral Hernia
Clinical Signs and Diagnosis
Rupture of the prepubic tendon causes a bilateral and complete loss of fore udder definition, and the fore udder points ventrally in continuum with the pendulous ventrally directed abdominal wall (Figure 9-2). The cow assumes a sawhorse stance because of exquisite pain in the abdomen and is reluctant to lie down (Figure 9-3). When the cow does lie down, she tries to assume lateral recumbency to avoid putting pressure on the ventrum. Abdominal distention usually is obvious. The owner usually observes overt or atypical extensive abdominal distention before rupture of the tendon, and cows (like mares) may have the rupture preceded by both extreme abdominal distention and a large plaque of ventral edema. These characteristics have been referred to as “impending rupture of the prepubic tendon” and are a grave sign.
Cattle with either condition may require assistance in calving because pain and inability to generate normal abdominal press may lead to dystocia. In addition, twins or hydrops may be apparent and further the chances of dystocia. Cows with rupture of the prepubic tendon should be salvaged or euthanized because they are in extreme pain. Cows with ventral hernias that do not appear to be in severe pain may be kept for the current lactation but should not be bred back. Cattle thought to be showing signs of “impending rupture” or those suspected to have hydrops should be induced to calve immediately or undergo elective cesarean section in an effort to prevent herniation or rupture of the prepubic tendon. Cattle with either condition that are recumbent and unable to stand or those already having secondary musculoskeletal complications should be euthanized.
Uterine Rupture
Treatment
Bactericidal antibiotics are indicated preoperatively and for at least 2 weeks postoperatively if repair has been successful. Intraperitoneal lavage with saline and antibiotics or very dilute Betadine is indicated and facilitated when the uterine tear is repaired through a flank approach. Further supportive therapy such as IV fluids, NSAIDs, or short-acting corticosteroids for cattle showing early signs of shock may be indicated. Prognosis is poor to guarded for cattle with uterine rupture.
Uterine Prolapse
Clinical Signs and Diagnosis
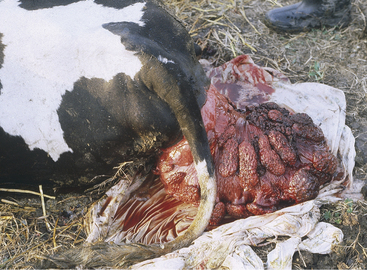
The clinical signs are dramatic and suffice for definitive diagnosis (Figure 9-4). Occasionally neophyte handlers or those privileged to have never seen the condition will confuse uterine prolapse with vaginal prolapse, but the sight of a fully prolapsed uterus is difficult to confuse with other conditions. Conspicuous placentomes on the exposed endometrium make the prolapsed uterus impossible to confuse with any other organ. The cow may appear healthy otherwise; this is often the case in primiparous cattle. Pluriparous cows with uterine prolapse often show varying degrees of hypocalcemia such as weakness, depression, subnormal temperature, anxiety, struggling or prostration, and coma. Tenesmus is common to most cases. Signs of shock should be differentiated from those of hypocalcemia because a small percentage of prolapse patients may develop hypovolemic shock secondary to blood loss (internal or external), laceration of the prolapsed organ, or intestinal incarceration within the prolapsed organ. Extreme pallor, a high heart rate, and prostration are grave signs in such cattle. Rarely the cow is found dead, especially when an unobserved calving has occurred. The prolapsed uterus often is heavily contaminated with bedding, feces, dirt, and placenta. Some bleeding is common from exposure injuries to the placentomes or endometrium. If the affected cow is able to stand and walk, the massive organ hangs near the hocks and can be stretched, traumatized, or lacerated as it flops back and forth against the rear quarters.
Treatment

Figure 9-5 Adult cow positioned in sternal recumbency for replacement of uterine prolapse
(Courtesy Dr. Nigel Cook.)
Retained Fetal Membranes
Etiology
Recent evidence strengthens the hypothesis that RFM is mediated by impaired neutrophil function beginning in the late dry period. Reduced neutrophil migration toward tissue extracts of placentomes can be detected as long as 2 weeks before calving in cows that go on to develop RFM. Other neutrophil functions, such as oxidative burst (a component of neutrophil bacterial killing action) are also impaired in these cows. Impaired neutrophil function has also been recorded in hypocalcemic cows. Indeed, many of the etiological factors associated with RFM have also been correlated to impairment of neutrophil function, including vitamin and mineral deficiencies, heat stress, or exogenous corticosteroid administration. The poor neutrophil function in affected cows extends into the postpartum period and probably mediates most of the complications usually associated with RFM.
Clinical Signs and Diagnosis
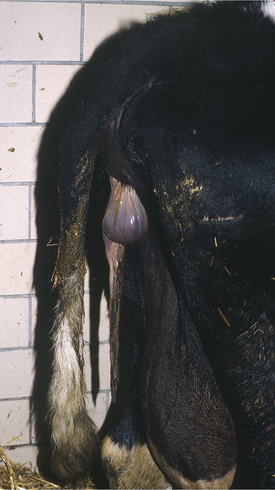
Clinical signs are obvious when the fetal membranes protrude from the vulva or hang ventral from the vulva to the escutcheon, rear udder, or hocks (Figure 9-6). The condition is less apparent when the membranes are retained within the uterus or only project into the cervix or vagina and require a vaginal examination to be detected. Other clinical signs are completely dependent on evolution of associated diseases. Metritis is the most common secondary complication, and clinical signs of metritis or endometritis are identical to those discussed in the metritis section. Secondary metabolic conditions may be linked directly to RFM when metritis exists or merely concurrent when the metritis is insignificant. As previously mentioned, mastitis, metabolic diseases, ascending urinary tract infections, and displaced abomasum may be associated with RFM complicated by metritis or, in the case of infections, because of less than optimal neutrophil function.
Treatment
A fascinating historical summation of treatment for RFM in cattle involving thousands of patients treated over several decades by the Ambulatory Clinic of the New York State Veterinary College is detailed in Roberts’ text. A summary of these data would suggest that less invasive, less manipulative treatments in association with intrauterine or systemic antibiotics (as indicated by the individual patient’s need and degree of metritis) progressively lessened the mortality rate for cattle with RFM. Cattle that resolve RFM and cycle normally should have fertility rates comparable with unaffected herdmates when breeding is begun at 90 days but may require adjunctive therapy in herds that begin breeding at 50 to 60 days as is common today.
Acute Puerperal Metritis
Etiology
Postparturient metritis is extremely common in dairy cattle and is best thought of as a spectrum of diseases depicted as a biologic bell-shaped curve. The most severe manifestation of metritis, perimetritis, implies full-thickness infection of the uterus with subsequent serosal leakage resulting in pelvic and peritoneal complications. Perimetritis is rare, potentially fatal, and most often follows severe dystocia. Septic metritis (acute puerperal or postpartum metritis, toxic metritis) refers to a severe puerperal uterine infection of the endometrium and deeper layers that results in systemic signs of toxemia. Puerperal metritis usually occurs from 1 to 10 days postpartum. “Metritis” is sometimes used as a general term for postpartum uterine infections of the endometrium or endometrium and deeper layers that may or may not cause systemic signs but may have implications for future reproductive performance. Infectious causes of reproductive failure such as brucellosis, leptospirosis, trichomoniasis, and campylobacteriosis may also cause varying degrees of metritis, but this discussion will be limited to conventional postpartum metritis.
On rectal palpation, early postpartum uteri have good muscular tone and may have palpable longitudinal ridges associated with muscular contraction. Pluriparous cows usually have uteri too large to retract manually or to palpate fully before day 10 to 14. Some primiparous cows may have sufficient involution to allow full definition of the uterus per rectum by days 10 to 14. Rectal palpation may not, however, be the best means to detect abnormalities in uterine involution during the first 14 days following parturition, and veterinarians should not hesitate to perform clean vaginal examinations and a vaginal speculum examination as adjuncts. The onset of estrus causes a remarkable difference in the size of the organ in most cattle, and slightly cloudy or clear mucus may be massaged from the uterus and cervix at this time. Most grossly detectable uterine involution is completed by 25 to 30 days postpartum, although the uterine horns still may feel thicker or more doughy than normal until days 35 to 40. The process of involution is completed more quickly in primiparous cattle than older cattle, in cattle free of metritis, cattle without RFM, cattle without metabolic disease, and cattle that have not had dystocia. Normally during a vaginal examination at 2 days postpartum, a hand cannot be passed through the cervix, and by day 4, only two fingers can be passed into the cervix. Cervical involution may be delayed by dystocia, cervical trauma, or RFM.
Clinical Signs and Diagnosis
Clinical signs usually suffice for definitive diagnosis of septic metritis. Ancillary data support an overwhelming infection as evidenced by a degenerative left shift in the leukogram and elevated fibrinogen values. Acute recruitment of neutrophils to the uterus out of the bloodstream weakens the patient’s cellular defenses against other infections—especially mastitis. A mild metabolic alkalosis is anticipated in cattle with gastrointestinal stasis. Differential diagnosis includes consideration of septic mastitis, peritonitis of any source, and acute pyelonephritis. Cultures of the uterine fluid never are contraindicated but, frankly, seldom are performed in dairy cattle. It is assumed that A. pyogenes, anaerobes such as F. necrophorum and Prevotella melaninogenica, and other organisms are present. Coliforms are common following dystocia or RFM and could cause additional endotoxin production. Clostridial organisms also have been identified in some septic metritis patients, and Clostridium tetani has been identified rarely in the uterine flora from cattle that develop tetanus secondary to septic metritis. If cultures are elected, both aerobic and anaerobic testing should be performed.
Ancillary data also may be helpful when the condition must be differentiated from retroperitoneal inflammation caused by vaginal perforations or extensive pelvic hematoma or inflammation secondary to dystocia. Abdominal paracentesis will indicate peritonitis based on increased protein and white blood cell counts in perimetritis patients but may be normal or have only moderately increased protein in retroperitoneal conditions. A complete blood count usually shows a degenerative left shift when perimetritis is peracute or acute. Neutrophilia is possible in subacute or chronic cases. Serum albumin tends to be decreased because of extensive protein loss into the uterus and peritoneal cavity in severe perimetritis cases (Figure 9-7). Vaginal examination may be indicated to rule out purely vaginal conditions and should be performed very gently following epidural anesthesia to avoid “malignant tenesmus,” which is a constant straining fueled by movement of air into the rectum.
Cattle that survive show slow but continual improvement as evidenced by a gradual return to normal temperature, normal heart rate, and return of appetite. Long-term (3 to 4 weeks) antibiotic therapy is indicated, and attempts to palpate the reproductive tract should be avoided for 3 to 4 weeks so as not to disturb adhesions. Surviving cows will always have extensive adhesions of the reproductive tract in the caudal abdomen and pelvis. It is best to allow complete sexual rest; prostaglandins should be administered at 14-day intervals to encourage uterine evacuation. Evacuation obviously is hampered by uterine adhesions. The cow should be assessed by rectal palpation once monthly. Despite the presence of extensive adhesions, surviving cattle eventually may resolve many of the adhesions over a 5- to 6-month period and conceive. Frequently these cows only “work on one side”—meaning that one uterine horn, uterine tube, or ovary (or all these) continues to be confined by adhesions. Therefore such a cow may conceive only when an ovulation occurs on the healthy or nonadhered ovary. Careful evaluation of the caudal reproductive tract and the presence of mature fibrous adhesions within the pelvic canal are critical before a decision as to breeding the cow back is made. Some cattle with persistent adhesions may be able to conceive and carry a pregnancy to term but should undergo elective cesarean section rather than attempting a natural or assisted vaginal delivery.
Clinical Endometritis
Evaluation of treatment options has been limited by lack of a widely accepted definition of clinical endometritis and a failure to concentrate on reproductive outcomes. Thus intrauterine infusion was the mainstay of treatment of bovine endometritis for decades. In spite of this, there was no convincing evidence that this mode of therapy had any beneficial effect on future reproductive performance of affected cows. In the face of mounting public concern about medicinal remedies in edible animal products, it is hard to justify antimicrobial treatment of dubious efficacy. It is interesting to note that the first words of skepticism regarding intrauterine infusions were raised in 1956 by Roberts. The primary alternative to intrauterine therapy has been systemic administration of PGF2α. Unfortunately, evidence for this approach is not entirely convincing either.
Subclinical Endometritis
Intrauterine Therapy
Intrauterine antibiotics have fallen largely into disfavor despite having been used for decades in the treatment of metritis in cattle. Intrauterine antibiotics also are often absorbed from the uterus to establish blood and milk levels that cause concern for milk residues and discard, resulting in significant economic losses. Absorption is more likely from healthy uteri, at the time of estrus, and following uterine involution. Certain antibiotics may interfere with phagocytic cell function, may be made inactive by beta-lactamase-producing bacteria, may irritate the endometrium, or may not work well in the relative anaerobic state thought to exist in the uterus. Perhaps the greatest impediment to using intrauterine antibiotics lies in the fact that large amounts of uterine fluid may simply overwhelm or inactivate small doses of locally administered antibiotics. Many intrauterine treatments may be compared to a “drop in the ocean” when used in severe metritis cases. Cephapirin, a first-generation cephalosporin, is available in some countries in a formulation designed specifically for intrauterine administration (Metricure, InterVet). It is not available in the United States. In several trials it has been found to be beneficial for treatment of clinical and subclinical endometritis. Its use in acute puerperal metritis has not been reported.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree