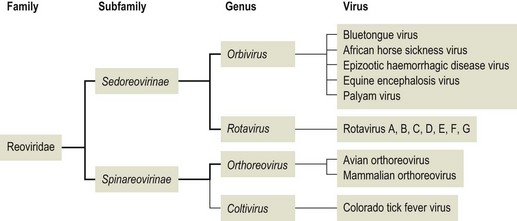
Chapter 55 The term reovirus is an acronym derived from respiratory, enteric and orphan viruses. Original isolations of these viruses were from respiratory and enteric samples but without associated clinical disease, hence the term orphan. Members of the Reoviridae are icosahedral in structure, 60–80 nm in diameter, non-enveloped and possess a one-, two- or three-layered capsid. Two morphological forms are recognized, reflected in the division of the family into two subfamilies: Spinareovirinae (Fig. 55.1) and Sedoreovirinae (Fig. 55.2). Viruses in the subfamily Spinareovirinae have spikes or turrets at the vertices of the icosahedral virus while members of the subfamily Sedoreovirinae lack such projections. The development of infectivity by some reoviruses requires the action of a protease on the outer capsid to produce infectious or intermediate subviral particles (ISVPs). The genome of the virion comprises 10–12 segments of linear, double-stranded RNA. Genetic reassortment occurs readily between members of the same genus. Replication occurs in the cytoplasm of host cells and frequently gives rise to intracytoplasmic inclusion bodies. The family contains 15 genera, of which members of the genera Orthoreovirus, Rotavirus and Orbivirus infect animals and man (Fig. 55.3). Members of the genus Coltivirus are associated with infections of arthropods, rodents and man while viruses belonging to the genus Aquareovirus infect fish. The genera Fijivirus, Phytoreovirus, Seadornavirus, Idnoreovirus, Cypovirus, Dinovernavirus, Cardoreovirus, and Oryzavirus contain viruses of arthropods and plants, while the genera Mycoreovirus and Mimoreovirus contain viruses of fungi and algae respectively. The viruses are moderately resistant to heat, organic solvents and non-ionic detergents. Reoviruses and rotaviruses are stable over a wide pH range while orbiviruses lose infectivity at low pH. Orthoreoviruses are widespread in nature and have been isolated from many mammalian and avian species (Table 55.1). Infections are generally considered nonpathogenic except in poultry and rodents. Mammalian and avian orthoreoviruses have distinct group antigens. Orthreoviruses may also be divided into subgroups on the basis of their ability to induce cell–cell fusion from within, termed fusogenic and non-fusogenic orthoreoviruses. Typically, avian isolates are fusogenic while mammalian isolates are non-fusogenic. Atypical, fusogenic orthoreoviruses have been isolated from baboons, flying foxes and snakes. Phylogenetic analysis based on the nucleotide sequences of the three S-class genome segments encoding the major sigma class core, outer capsid, and non-structural proteins indicates at least four genogroups or species of orthoreoviruses (Duncan 1999), five species are currently recognized. Rotaviruses are a significant worldwide cause of human infantile gastroenteritis and of acute diarrhoea in intensively reared farm animals. Transmission of orthoreoviruses and rotaviruses is by direct and indirect contact with contaminated faeces. A number of important animal diseases are caused by orbiviruses. Twenty-two serogroups or species of orbiviruses are currently recognized, as well as a number of unclassified viruses. The groups include serotypes and antigenic complexes. Both African horse sickness and bluetongue are categorized as listed diseases by the Office International des Epizooties (OIE). Epizootic haemorrhagic disease virus and Ibaraki virus of deer and cattle respectively are similar to bluetongue virus in infection and disease patterns. Infection with equine encephalosis virus has only been recognized in southern Africa and Israel. Serological evidence suggests widespread infection with this virus but acute disease is sporadic in occurrence. Orbivirus transmission involves arthropod vectors, primarily Culicoides species. Table 55.1 Members of Reoviridae of veterinary significance Reoviruses are frequently recovered from the gastrointestinal tract of clinically normal birds. However, under certain circumstances they may give rise to disease directly or enhance the pathogenicity of other infectious agents. Avian othoreoviruses have been linked with a diverse range of conditions including arthritis, tenosynovitis, chronic respiratory disease and enteritis. More than nine serotypes can be distinguished using serum neutralization tests. Transmission is mainly horizontal by the faecal–oral route but vertical transmission through the embryonated egg is also possible. Reoviruses are an important cause worldwide of viral arthritis/tenosynovitis in meat-type chickens between four and 16 weeks of age. Morbidity is typically less than 10%. The condition principally presents as lameness. Rupture of the gastrocnemius tendon may occur such that the bird is unable to bear weight on the affected leg. Affected birds lose weight and may die of starvation as a result of difficulties in reaching feed and water. The lesions are not pathognomonic as similar lesions can be induced by Mycoplasma synoviae and Staphylococcus aureus. Diagnosis of reovirus involvement is best carried out by virus isolation. The specimens of choice are hock articular cartilage and affected tendons. Synovial fluid from the hock joint is also suitable but gives lower rates of virus recovery. Suspensions of suspect material can be inoculated into the yolk sac of embryonated hens’ eggs or onto monolayers of chick embryo liver cells. Viral antigen may be detectable by immunofluorescence in cryostat sections of affected tendons. Reverse transcription-PCR protocols have been described for the detection and differentiation of avian orthoreoviruses (Liu et al. 1999, Caterina et al. 2004, Liu et al. 2004). Serological testing is not particularly useful due to the high prevalence of subclinical infections. It may be useful on a flock basis to determine the immune status of parent flocks and young chicks. The severity of infection varies greatly and is influenced by several factors including age, viral virulence, colostral immunity, overcrowding and the presence of other enteric pathogens. The incubation period is usually less than a day. Following oral ingestion, virus passes largely unaffected through the acidic medium of the stomach and infects the columnar enterocyctes lining the apices of the villi of the small intestine. In young animals the rate of replacement of enterocytes is relatively slow and affected villi become shortened and covered by cuboidal replacement cells. These changes are most pronounced in the proximal small intestine. These immature cells have reduced levels of disaccharidases and impaired glucose-coupled sodium transport. In addition a non-structural protein of the virus NSP4 has been shown to act as an enterotoxin affecting the co-transport of sodium ions and galactose/glucose (Lorrot & Vasseur 2007). Undigested lactose in milk forms a substrate for bacterial proliferation and exerts an osmotic effect. As a result, increased amounts of fluid are retained in the lumen of the gut and diarrhoea ensues. Affected animals are anorexic, depressed and produce light-coloured semi-liquid or pasty faeces. In uncomplicated cases animals frequently recover within three to four days without treatment. However, concurrent infection with other enteric pathogens such as Escherichia coli, Salmonella species and Cryptosporidium species may give rise to severe diarrhoea and significant mortality. Suitable samples include faeces and intestinal contents. • Negative-contrast electron microscopy is rapid and capable of detecting combined virus infections. However, it requires a large concentration of virus particles (106 per gram of faeces) and specialized equipment. The sensitivity of the procedure can be improved by using immunoelectron microscopy. • Viral antigen can be detected in faeces by ELISA or latex agglutination. The antisera employed in these tests is usually reactive with group A rotaviruses only. These assays are available commercially. Immunofluorescence can also be used to detect viral antigen in smears or cryostat sections of affected small intestine. • Sodium dodecyl sulphate-polyacrylamide gel electrophoesis (SDS-PAGE) has been used to detect the RNA segments of rotaviruses in clinical samples (Herring et al. 1982). The sensitivity of this procedure is similar to that obtained using electron microscopy. Differentiation of the different rotavirus serogroups is possible using the electrophoretic patterns obtained. • Rotaviruses are difficult to isolate in vitro. However, the presence of low concentrations of trypsin in the growth medium will facilitate viral uncoating and improve viral replication. • Reverse transcription PCR protocols are available for the detection (Gouvea et al. 1990) and genotyping of rotaviruses (Gentsch et al. 1992, Gouvea et al. 1994, Iturriza-Gómara et al. 2000).
Reoviridae
Virus
Natural host
Significance of infection
Mammalian orthoreovirus
Wide range of species
Four serotypes. Associated with mild enteric and respiratory disease. Significance often dependent on presence of secondary infectious agents
Avian orthoreovirus
Chickens, turkeys and other avian species
Multiple serotypes. Important cause of viral arthritis/tenosynovitis in chickens and possibly turkeys
Rotavirus A to G
Neonates of many intensively reared domestic species including calves, lambs, piglets, foals and poultry
Mild to severe diarrhoea dependent on age, colostral intake, accommodation and concurrent infections. Rotavirus E is associated with pigs while rotaviruses D, F and G are associated with birds
African horse sickness virus
Equidae
Non-contagious arthropod-borne infection. Endemic in Africa south of the Sahara. Mortality rate often high in susceptible horses. Listed disease
Bluetongue virus
Sheep, cattle, goats, deer, antelopes
Arthropod-borne infection of ruminants. Clinical disease highly variable in severity. Severe disease seen in fine wool and mutton breeds of sheep and in certain deer species. Teratogenic virus. Clinical disease is uncommon in cattle. Listed disease
Epizootic haemorrhagic disease virus (EHDV)
Deer, cattle, buffalo
Arthropod-borne, principally Culicoides species. Important disease of deer in North America. Clinically similar to bluetongue. Infection of cattle is usually subclinical or mild. Seven serotypes
Ibaraki virus
Cattle
Acute febrile disease similar to bluetongue. Described in southeast Asia. Member of the EHDV group (EHDV-2)
Equine encephalosis virus
Horse
Seven serotypes. Only reported in South Africa and Israel. Majority of infections are subclinical with sporadic cases of an acute fatal, disease characterized by brain oedema, fatty liver degeneration and enteritis
Colorado tick fever virus
Arthropod-borne, primarily ticks but also mosquitoes. Prolonged viraemia in rodent species (reservoir host)
Humans and other mammals may be dead-end hosts. May cause encephalitis in children
Palyam virus
Cattle
Large serogroup of viruses. Arthropod-borne. Associated with abortions and teratogenicity. Recorded in southern Africa, southeast Asia and Australia
Avian orthoreoviruses
Rotaviruses
Pathogenesis
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Reoviridae
Only gold members can continue reading. Log In or Register to continue