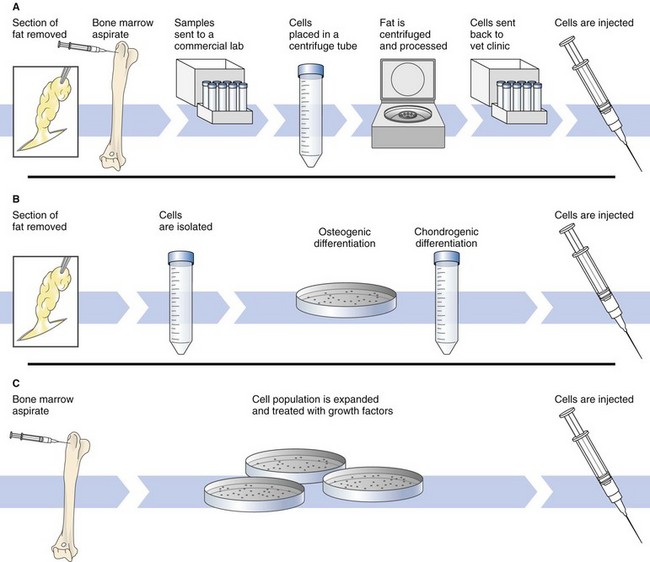
Chapter 14 Regenerative medicine is the process of creating living, functional tissues to repair or replace tissue or organ function lost because of disease, damage, or congenital defects. It incorporates numerous biomedical approaches including tissue engineering, stem cell therapy, and medical devices. Stem cells are cells with the ability to divide for indefinite periods in culture and to give rise to specialized cells. Embryonic stem cells (ESCs) are primitive or undifferentiated cells derived from a 5-day embryo that are capable of dividing without differentiating for a prolonged period in culture and are known to develop into cells and tissues of the three primary germ layers. Embryonic stem cell lines are embryonic stem cells that have been cultured under in vitro conditions that allow proliferation without differentiation for months to years. Bone marrow or “adult” stromal cells are a population of cells found in bone marrow that are different from blood cells, a subset of which are multipotent stem cells, able to give rise to bone, cartilage, and fat cells and able to support formation of blood cells. Mesenchymal stem cells (MSCs) is a term that is currently used to define nonblood adult stem cells from a variety of tissues, although it is not clear that MSCs from different tissues are the same. Stromal cells are connective tissue cells found in virtually every organ. In bone marrow, stromal cells support blood formation. Induced pluripotent stem cells (iPSCs) are the product of reprogramming a somatic cell into an embryonic stem cell (ESC)-like state. This technique, first reported in 2007, provided a new approach to the generation of ESC-like cells (Takahashi et al, 2007). Platelet-rich plasma (PRP) is an autogenous fluid concentrate composed primarily of platelets and white blood cells used to accelerate healing of tendon, ligament, bone, and other tissues. The American Red Cross defines PRP as 5.5 × 1010 platelets/50 ml (Dohan Ehrenfest et al, 2009). Protein-rich gel (PRG) is formed by combining PRP with thrombin and calcium chloride to form a semisolid material. The history of regenerative medicine dates back many centuries, even being considered in the Middle Ages (Weaver and Garry, 2008). In the 20th century, Medawar focused on the rejection of skin grafts in mice. In 1958, Jean Dausset characterized the first human histocompatibility antigens, the human leukocyte antigens (HLAs), which led to the understanding that the ability of the immune system to recognize self from the nonself was a cellular phenomenon and that acceptance of an allogeneic graft or transplanted organ correlated with the level of HLA matching between the recipient and donor. Although in the 1950s several bone marrow transplants were attempted, it was not until 1968 and 1973, respectively, that the first related and unrelated bone marrow transplants were successfully performed (Weaver and Garry, 2008). Although the concept of stem cells was introduced over a century ago by Alexander Maximow, modern stem-cell research began in 1963 when Till, McCullough, and Siminovitch established assays to detect hematopoietic stem cells (HSCs). Using irradiated murine hosts, researchers found that bone marrow transplants resulted in macroscopic colonization of the spleen, the cells of which possessed innate capacity for proliferation, differentiation, and self-renewal—the hallmarks of a stem cell pool (Weaver and Garry, 2008). This work eventually confirmed the existence of stem cells and marked the beginning of modern-day stem-cell research. The regulatory, moral, and ethical issues associated with the use of embryonic stem cells has limited their use in humans; thus, the focus of this discussion will be on adult, bone marrow–derived stem cells. A number of researchers have been working to create genetically defined/animal-specific ESCs that could be expanded in vitro, differentiated into the cell type of choice, and then used for tissue replacement therapy or for in vitro research including drug and toxicology screens (Schneider et al, 2010) (Fig. 14-1). These cells could then be used for preclinical studies evaluating the safety of ESC-derived tissues and potentially could lead to the development of a canine gene knockout ESC line. Strategies that utilize these cells for therapy are known as cell-based therapies. The best-known clinical application of cell-based therapies is bone marrow transplantation to repopulate the marrow and restore blood cells after high-dose chemotherapy or radiotherapy. However, because the need for donated organs and tissues often used to replace ailing or destroyed tissue far outweighs the available supply, the use of stem cells directed to differentiate into specific cell types is becoming increasingly important. Stem cells offer the possibility of a renewable source of replacement cells and tissues to treat many diseases including spinal cord injury, burns, heart disease, diabetes, osteoarthritis, and rheumatoid arthritis. Blood transfusions, fresh frozen plasma, platelet-rich plasma, cryoprecipitates, and blood substitutes are discussed on page 36 and Table 4-5. There are three basic methods for obtaining and preparing stem cells for administration. (1) The simplest technique is to collect fat or bone marrow and centrifuge it to concentrate the cells. This produces a mixture of both stem and nonstem cells and generally results in a relatively low yield of stem cells. Techniques that rely on centrifugation generally produce a dilute MSC population (1 in 10,000 to 1,000,000 of the nucleated cells are stem cells). This is the method currently being used for production of stem cells for veterinary patients. In humans, flow cytometry, based on MSC reactivity to a number of antibodies, is increasingly being used to select for expressed antigens to remove nonmesenchymal cells from the sample. For instance, antibodies against CD34, a surface marker found on hematopoietic cells, are frequently used to identify and remove nonmesenchymal cells from a marrow culture. (2) A second technique is to isolate the cells and treat them with growth factors/cytokines to stimulate their differentiation into the cell type of interest (e.g., chondrocyte, cardiac myocyte) (Fig. 14-2). Osteogenic differentiation of MSCs can be induced in vitro by treating a monolayer culture with a pro-osteogenic cocktail such as dexamethasone, ascorbic acid-2-phosphate, and beta-glycerophosphate. For chondrogenic differentiation, bovine insulin, transferrin, selenious acid, linoleic acid, bovine serum albumin, sodium pyruvate, proline, L-glutamine, or TGF-beta1 may be added. Recently, MSCs have been shown to have the ability to form muscle cells and cardiomyocytes when treated with the demethylating agent 5-azacytidine. This technique may still result in a relatively low yield of stem cells (albeit predifferentiated cells). (3) The most complex technique (which is considered to be the gold standard) is to obtain the cells, expand the cell population in culture, and then treat them with growth factors. This latter technique, in which an expanded cell population is administered, results in significantly greater cells being given. Billions of MSCs can be produced from a small bone marrow aspirate. Numerous factors may affect the number and function of stem cells, including the age and physical status of the donor, tissue source (i.e., fat, bone marrow), method of harvest, and manipulation, handling, and storage of the sample. It has been shown that the proliferation and chondrogenic differentiation capabilities of a 4-month-old dog are higher than those in a 12-month-old dog (Li et al, 2007). MSCs may be autologous (from self), allogeneic (from a donor of the same species), or xenogeneic (from another species). Although human MSCs have been shown to maintain their multipotential capacity after transplantation into sheep, the immune response to xenografts (adult) is much more vigorous than to allografts (Yang, 2004) because of the greater antigenic differences that exist among different species than within a species. Thus, most current research is being done on allogeneic and autologous MSCs. MSCs represent an advantageous cell type for allogeneic transplantation because evidence suggests that MSCs are immune-privileged with low MHC I and no MHC II expression (Uccelli et al, 2006), therefore reducing risks of rejection and complications for transplantation. Although MSCs may be protected from the immune system, many authors believe that autologous MSCs are preferred over allogeneic stem cells in this respect. There are advantages and disadvantages of each approach. The Food and Drug Administration (FDA) does not currently regulate autologous stem cells in veterinary medicine because they are minimally manipulated (i.e., not expanded in cell culture) and are given back to the same animal from whom the tissue (e.g., bone marrow, adipose) came from. Lack of federal regulation makes commercial production of allogeneic stem products substantially cheaper and easier than allogeneic products, which are regulated. However, controversy exists as to the number of stem cells that are being returned to the patient and their activity when autologous processes are used. Several studies have provided evidence that the ability of stem cells to respond to environmental demands may be diminished during aging (Rao and Mattson, 2001); thus, patients that are old or sick and perhaps most in need of stem cells may have the fewest or least functional stem cells with which to repair their own tissues (Chambers et al, 2007). Several companies are currently marketing autologous stem cells in veterinary medicine for treatment of joint, bone, and ligament injuries of horses and dogs. Use of allogeneic MSCs is currently being studied by numerous groups and has been widely tested in clinical trials of cardiovascular, neurologic, and immunologic disease in humans, with encouraging results (Malgieri et al, 2010). There are examples of MSC utilization in the repair of ischemic heart disease, chronic kidney disease, and inflammatory lung disease (e.g., acute lung injury, chronic obstructive pulmonary disease, asthma, and pulmonary hypertension) and in the treatment of chronic wounds; however, much research is still needed before using these cells is considered a routine part of clinical practice. Of concern is whether differentiation of MSCs will lead to induced immunogenicity and thus limit their long-term benefit in diseases such as myocardial infarction (Huang et al, 2010). MSCs may be administered with or without culture expansion. Because only a small number of the nucleated cells in bone marrow are capable of differentiating into bone, cartilage, or muscle, administration of expanded cells may be most beneficial.
Regenerative Medicine and Stem Cell Therapy
General Principles and Techniques
Stem Cells and Regenerative Medicine
Mesenchymal Stem Cells
Autologous versus Allogeneic Stem Cells
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Regenerative Medicine and Stem Cell Therapy