Chapter 23 Rational Use of Reproductive Hormones
Physiologic Principles of Reproductive Hormones
Hypothalamic Hormones
Gonadotropin-Releasing Hormone and Its Analogs
Gonadotropin-releasing hormone (GnRH) is a highly conserved hypothalamic decapeptide with the same amino acid sequence in all mammals. After puberty GnRH is released in a pulsatile manner from the hypothalamus, traverses the hypothalamic–hypophyseal portal system, and activates anterior pituitary gonadotroph receptors. The pituitary responds by releasing luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in a pulsatile pattern.1 Stimulation of the pituitary by GnRH must be in pulsatile form for repeated release of LH and FSH; after receptor activation, GnRH is rapidly deactivated and cleared. The frequency and amplitude of GnRH pulses vary depending on the phase of the reproductive cycle. The frequency of GnRH pulsatile release in primates during folliculogenesis is every 70 to 90 minutes.2 It is this natural pulsatile secretion of GnRH and its short biological half-life that cause difficulties when attempting to use GnRH as a pharmaceutical. Investigations with specialized infusion devices for pulsatile delivery have successfully augmented fertility in a variety of species,3–5 but the clinical application of such a method lacks practicality for routine use in small animal patients.
GnRH analogs are synthetically prepared substances that differ from GnRH by various amino acid substitutions in the peptide sequence. Analogs with a few amino acid substitutions can act as GnRH agonists because of their increased binding affinity and decreased clearance compared with GnRH. Heavily substituted analogs can cause receptor blockade and have an antagonist function. The result is a suppressive effect on the pituitary–gonadal axis. This axis is easily downregulated such that frequent or high dosing of a GnRH agonist will suppress the release of LH and FSH also.6 GnRH agonists and antagonists thus may have the same ultimate physiologic effect.
Oxytocin
A nonapeptide hormone synthesized by neurons in the hypothalamus, oxytocin is transported axonally to the posterior pituitary, where it is stored. This peptide hormone is released from the posterior pituitary into the general circulation after appropriate neural stimulation. Its primary effects are on mammary tissue and the myometrium. The effects of oxytocin on the milk let-down reflex and parturition have been well described.7 The ability of oxytocin to induce myometrial contraction is enhanced by prior estrogen sensitization to “prime” the myometrium for maximal response.8 The half-life of oxytocin in the blood is short, approximately 1.5 minutes; thus secretion pulse frequency and amplitude are important for its physiologic effects. The release of neurotransmitters may alter the amplitude and frequency of oxytocin release; for example, dopamine enhances burst frequency and amplitude, whereas cholinergic antagonists may be inhibitory.9
Pituitary Gonadotropins
Luteinizing Hormone and Follicle-Stimulating Hormone
The pituitary gonadotropins LH and FSH are relatively large glycoproteins, each consisting of two covalently bound structural subunits (α and β). Within a species the amino acid sequence of the α-subunits are identical for all anterior pituitary glycoproteins, with high sequence homology existing across species. The β-subunits are specific for individual hormones (i.e., thyroid-stimulating hormone) and provide the functional specificity of each. The overall size of these glycoproteins precludes economic synthetic production of these hormones.8
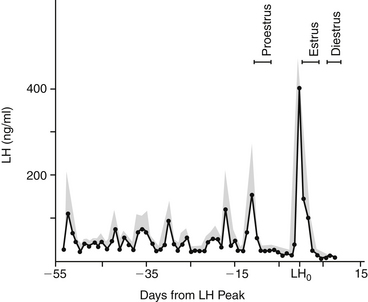
The secretion patterns of these hormones differ depending on the species and the phase of the ovarian cycle. A unique pattern of LH secretion in the bitch has been documented10 (Figure 23-1). Serum concentration of LH is at basal levels during anestrus. Significant increases in both the amplitude and frequency of LH pulsatile release occur before proestrus. The frequency of LH pulsatile release during anestrus is 3 to 7 hours; before proestrus LH pulse frequency is 60 to 120 minutes.11 This increase in LH release before the onset of proestrus is likely involved with termination of the anestrus phase.1 The factors that lead to the LH increase at that time are unknown. Serum estrogen concentration at that time also decreases; estrogen production inhibits LH release. What causes the relative decrease in estrogen production at that time is also unknown.
Serum LH concentration returns to basal levels for most of proestrus (i.e., folliculogenesis). The increase in serum estrogen concentration during folliculogenesis contributes to an inhibition of LH release during that phase.1 When estrogen production decreases, the inhibition of LH release is discontinued. At that time a preovulatory surge in LH release (the preovulatory LH peak) occurs and is thought to trigger ovulation. The duration of the preovulatory LH peak is 1 to 3 days, after which LH secretion returns to a basal state in early diestrus. Additionally, LH is luteotrophic throughout most of the luteal phase in the bitch.
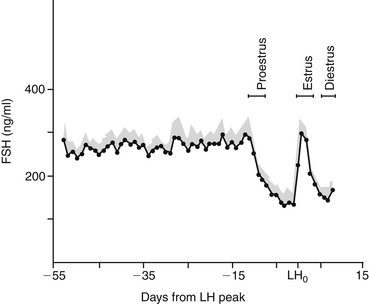
Similar to the pattern of LH secretion, FSH secretion also appears to be inhibited by relatively high concentrations of estrogen during proestrus. Inhibin, a modulary hormone released from the ovary during folliculogenesis, also inhibits FSH release during this phase. When estrogen secretion declines in late proestrus, FSH secretion surges to maximal levels before ovulation, in concert with the preovulatory LH peak1,10 (Figure 23-2). After this FSH surge, serum FSH concentration remains relatively high during diestrus and pregnancy. Interestingly, the bitch is also unique with regard to the pattern of FSH secretion during anestrus. During anestrus serum FSH concentration can be 50% to 100% of the concentration found at the preovulatory FSH peak and is 5 to 10 times higher than during proestrus.1 It is unclear why FSH produced during anestrus is unable to stimulate folliculogenesis. It has been postulated that perhaps the FSH measured during anestrus is in a biologically inactive form.1
The patterns of LH and FSH secretion in the queen have not been clearly determined. It has been documented that after adequate copulation LH release begins within minutes. Queens are induced ovulators; thus this response is expected. Apparent spontaneous ovulation, without copulation or other tactile stimulation, has been reported in the queen.12 The pattern of LH secretion during the apparent spontaneous ovulation has not been determined, however.
The release of LH and FSH is also pulsatile in response to GnRH in the male. Both glycoprotein hormones are necessary for spermatogenesis. Testicular interstitial cells bind LH and respond by increasing testosterone production. Activation of receptors on Sertoli cells by FSH promotes spermatogenesis and produces inhibin, a hormone that, as in the female, regulates FSH release by the pituitary. After neutering the loss of negative feedback inhibition causes serum FSH concentrations to increase dramatically.13 This has also been documented in cases of infertility resulting from primary testicular degeneration.14 Postcastration elevations in LH serum concentrations also occur, but overlap in measured values between intact and neutered dogs is possible.13
Prolactin
A relatively large polypeptide produced by the anterior pituitary, prolactin is luteotrophic in the bitch and queen.15,16 It is suspected that prolactin is also involved in the maintenance of the anestrus phase in the bitch.1 Prolactin is under negative control by dopamine such that the administration of a dopamine agonist will inhibit prolactin secretion. Inhibition of prolactin secretion can promote luteolysis during the second half of diestrus or pregnancy in the bitch15 and queen.16 Various dopamine agonists have been investigated for inducing abortion and shortening anestrus (i.e., estrus induction). Bromocriptine is a dopaminergic drug that has been used in various protocols. Its use has not gained acceptance, however, because it commonly causes vomiting and diarrhea. The highly potent dopamine agonists cabergoline and metergoline have fewer or no apparent side effects, respectively. The effect of metergoline to decrease prolactin secretion may primarily result from blockade of central serotonin receptors and may function as a dopamine agonist only at higher doses.17 Metoclopramide, more commonly used as a central serotonin antagonist, may be used to enhance prolactin release in the bitch and queen, and thus enhance milk let-down, through its central dopamine (D2) agonist effects.
Gonadal Steroids
Estrogen
Produced by the ovary during folliculogenesis, estrogens affect target tissues, causing vulvar edema, vaginal mucosal hyperplasia, sanguineous vulvar discharge in the bitch, and sexual attraction in both the bitch and queen. The bitch uniquely exhibits sexual receptivity when estrogen production decreases; serum concentration of estrogen peaks 1 to 2 days before the end of proestrus. A concurrent increase in progesterone is required for complete expression of sexual receptivity. Estrogen acts synergistically with progesterone to stimulate growth of endometrial and mammary glands, and estrogen priming may be important before the natural effect of oxytocin on the myometrium during parturition.8 Male production of estrogen occurs in both the Leydig and Sertoli cells, by way of de novo steroidogenesis in the Leydig cell and P450 aromatase conversion of testosterone alone in the Sertoli cell. Considerable interspecies variation occurs with respect to the relative ratios of estrogen produced by each cell. Excessive production of estrogens by Sertoli cell tumors and, less commonly, by other testicular or adrenal tumors results in male feminizing syndrome. Estrogens are involved in receptor sensitivity and feedback influence on the hypothalamic–pituitary–gonadal axis.
The adverse effects of estrogen (i.e., bone marrow aplasia, cystic endometrial hyperplasia/pyometra, infertility) have been documented in dogs after administration of any estrogen preparation.18,19 The unique sensitivity of dogs to estrogen toxicity may be due to the relatively weak binding affinity of sex-steroid binding proteins for estrogen in the dog. The result is a decrease in the inherent buffering mechanism that would otherwise regulate the amount of free hormone available to penetrate cell membranes. Estrogen toxicity can occur with exogenous or endogenous estrogens (e.g., Sertoli cell tumors, ovarian follicular cysts, and ovarian neoplasia).
Progesterone
As the main progestational hormone in both bitches and queens, progesterone is produced by corpora lutea just before and after ovulation. The bitch continues to produce progesterone during nonpregnant diestrus; serum concentrations of progesterone are indistinguishable in concentration or duration of production from that of pregnancy. The decline of progesterone and increase in prolactin production at the termination of diestrus cause the clinical manifestations of pseudopregnancy in the nonpregnant bitch. This is a demonstration of a normal physiologic occurrence that should not be confused with a pathologic state and in fact suggests normal ovarian function. There is some variation among bitches regarding the maximum amount of progesterone produced in midgestation (or mid-diestrus), with levels reaching 80 to 100 ng/mL in some individuals. The minimum serum progesterone concentration necessary to maintain pregnancy appears to be 2 ng/mL.20 The minimum amount of progesterone required to sustain pregnancy in the queen has not been determined; however, serum progesterone concentrations of less than 1 ng/mL for several days occurred before termination of pregnancy were observed in one study.16
Placental production of progesterone by the queen during the latter half of pregnancy had been suggested as a requirement to maintain pregnancy. It has been learned, however, that corpora lutea of the queen are necessary for progesterone production throughout pregnancy.21 During pseudopregnancy in queens (i.e., nonfertile ovulation), peak luteal activity appears to occur at days 10 to 15 and then declines to basal values by days 35 to 40.22
Synthetic progestational compounds (progestagens) are commercially available. Megestrol acetate has been marketed as a drug to suppress estrus or inhibit ovulation, depending on the time and dose of administration. The method by which progestagens inhibit folliculogenesis and ovulation is not precisely understood. It appears that although megestrol acetate will not decrease the serum concentration of LH that is already at low basal levels, it may be able to prevent the increases in LH that normally occur at the end of anestrus.1
Testosterone
Produced by the interstitial cells of the testes, testosterone is necessary for gonadal development, spermatogenesis, and libido in the male. The normal function of Sertoli cells to promote spermatogenesis depends on an intratesticular testosterone concentration that greatly exceeds circulatory levels.8 Pharmacologic administration of androgens, including testosterone, can induce infertility by negative inhibition of LH and FSH release, which are necessary for spermatogenesis. Testosterone is converted in the prostate to dihydrotestosterone (DHT), which promotes development of this gland and the eventually contributes to the anticipated formation of benign prostatic hyperplasia in mature dogs. DHT is an androgen with greater biological activity than testosterone.
Inhibitors of Gonadal Steroids
Tamoxifen is both an estrogen agonist and antagonist that has been used as adjunctive therapy for women with mammary carcinoma. The nature of its effect depends on tissue estrogen receptor type. Tamoxifen appears to have, at least in part, a direct estrogenic effect in the bitch. Some bitches treated with tamoxifen had observable vulvar edema; sanguineous vaginal discharge; and, in some cases, pyometra of the uterine stump.23 Tamoxifen and clomiphene, another antiestrogenic compound, have been used to promote superovulation in women, possibly by promoting an increase in endogenous FSH release. Tamoxifen has been used in the bitch as a mismate therapy but was associated with pyometra, endometritis, and cystic ovaries in a high percentage of bitches; was reliably effective only when given during the first 14 days of diestrus; and is not advised.24
Antigestagens are agents that inhibit the effect of progesterone by binding to and altering the progesterone receptor and have been investigated as abortion agents in humans (i.e., mifepristone, RU-486). Preliminary studies have documented the effectiveness of mifepristone to terminate pregnancy in bitches.25
Finasteride is another useful inhibitor of gonadal steroids. Testosterone is converted to DHT in the prostate by the action of the enzyme 5α-reductase. Trophic in its effect, DHT induces benign prostatic hyperplasia as a dog ages. Finasteride inhibits the action of 5α-reductase, thereby reducing DHT concentrations in the prostate. Finasteride may also alter prostatic angiogenesis and reduce hemospermia through a change in microvessel density. Developed for use in men with prostatic hyperplasia, finasteride has been investigated for successful similar use in dogs.26 Benign prostatic hyperplasia causing hemospermia can be detrimental to efforts to successfully freeze and thaw canine semen; otherwise, it is minimally problematic in the dog unless accompanied by infection or neoplasia.
Autacoids
Prostaglandin F2α
Prostaglandins, potent autacoids, have therapeutic indications in small animal theriogenology. Administration of prostaglandin F2α(PGF2α) induces a direct luteolytic effect in bitches and queens during pregnancy or diestrus. Induction of luteolysis depends on dose, frequency of drug administration, and stage of diestrus that the drug is administered. After day 30 of diestrus, PGF2α is reliably luteolytic in both the bitch27 and queen.22 Corpora lutea are relatively resistant to luteolysis by PGF2-alpha during the first 5 days of diestrus in the bitch.28 There is evidence that PGF2α will cause either a transient decrease in progesterone production or complete luteolysis when administered during early diestrus after day 6.28 In addition to a direct luteolytic effect, PGF2α also has a stimulatory action on the myometrium, promoting evacuation of uterine luminal contents. The drug is therefore effective as an abortifacient and for the treatment of open cervix pyometra in the bitch and queen.
Commercial Availability of Reproductive Hormones
Gonadotropin-Releasing Hormone And Its Analogs
Lack of availability is a major hindrance to widespread use of GnRH analogs to control reproduction in humans and animals. Analogs are expensive to produce and are often available only as investigational products for research studies; availability also varies throughout the United States. One marketed GnRH analog (an antagonist) is leuprolide (Lupron, TAP Pharmaceuticals, Deerfield, Ill.). The native GnRH hormone (gonadorelin, Rhone Merieux) is commercially available (Cystorelin, or Factrel, Fort Dodge Laboratories, Fort Dodge, Iowa). Implant formulations of GnRH agonists such as deslorelin have been shown to be effective for reversible long-term suppression of reproductive function in the male and female dog and estrus suppression in the queen29,30 and have been manipulated for estrus induction in the bitch.31
Indications for the Rational (Evidence-Based) use of Reproductive Hormones
Some reproductive disorders are common and well described in the literature (e.g., pyometra, dystocia). Others are either incompletely understood or are less common (e.g., estrus induction, luteal insufficiency) such that information can be difficult for the practicing veterinarian to obtain. The discussion presented here is intended to provide an overview of several reproductive problems of dogs and cats that commonly require veterinary intervention (Table 23-1). Many of these disorders are topics of current research, which is needed to better understand and manage them.
Table 23-1 Uses of Reproductive Hormones for Dogs and Cats
| Indication | Hormone Used | Considerations |
|---|---|---|
| Estrus induction | See Table 23-3 | |
| Induction of ovulation | ||
| Ovulatory failure | hCG or GnRH to induce ovulation | Difficult to accurately determine follicular maturity to administer at correct time |
| Ovarian cysts | hCG or GnRH to induce luteinization | Often ineffective, may require ovariectomy need to differentiate neoplasia |
| Vaginal hyperplasia | hCG or GnRH to hasten ovulation | Not proven to shorten time for spontaneous recovery |
| Luteal insufficiency | Progesterone to maintain gestation | Potentially teratogenic, inhibits lactation development, luteal insufficiency is rare; need diagnosis before treatment attempted |
| Estrus prevention | Mibolerone: unpredictable and unavailable, irregular return to estrus after withdrawal; megestrol acetate: potential for infertility, pyometra; requires compounding Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|