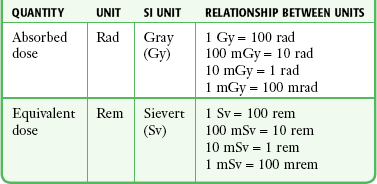
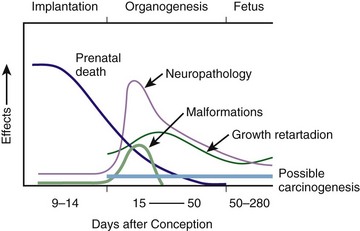
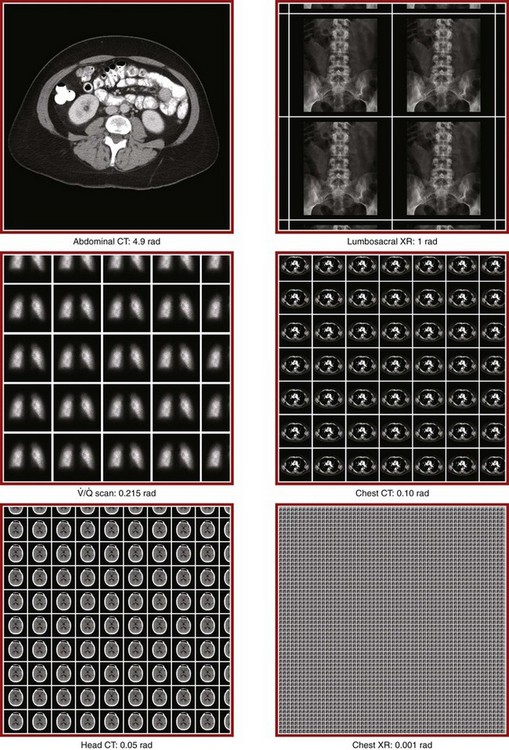
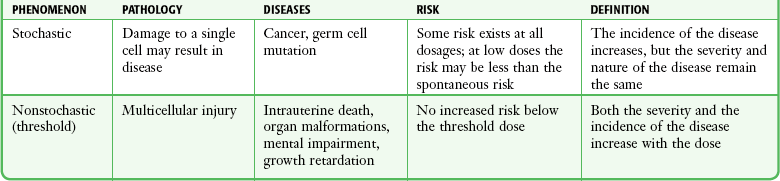
Chapter 72 The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) published a sentinel alert1 in August 2011 highlighting the radiation risks of diagnostic imaging in all patients, not just pregnant patients. This alert underscores the fact that x-rays are officially considered a carcinogen. Over the past 2 decades, total exposure to ionizing radiation has nearly doubled. Published reports estimate that the incidence of cancer secondary to radiation is 0.02% to 0.04%. In utero exposure of the embryo or fetus to radiation generally causes great, but largely unnecessary anxiety in patients, their families, and the clinician. Much of this anxiety is secondary to a general misconception that any exposure to radiation is harmful and will result in an anomalous fetus. More often than not, clinicians themselves add to the confusion and fear by providing exposed women with erroneous information. Many clinicians, nurses, and even radiologists are ignorant of the qualitative and quantitative effects of ionizing radiation.2 Multiple surveys in the literature reveal clinicians’ dearth of knowledge about radiation exposure. This misinformation could lead to inappropriate abortions and litigation. For example, in Greece after the Chernobyl disaster, 23% of pregnancies were terminated because of unsubstantiated fears of teratogenicity.3 A better understanding of the true risk estimates may help alleviate this fear. It is widely held and published that concerns about the possible effects of exposure to ionizing radiation should not prevent medically indicated diagnostic procedures from being performed on the mother. It is not standard of care to withhold necessary radiologic studies because of fear of fetal injury from diagnostic studies. According to the American College of Radiology, “No single diagnostic x-ray procedure results in radiation exposure to the degree that would threaten the well being of the preembryo, embryo, or fetus.”4–6 This remarkable statement helps put into perspective the effects of diagnostic radiation exposure on pregnancy. Standard diagnostic radiologic procedures performed in the ED are not associated with significant proven fetal risks. A clear understanding of these risks enables clinicians to make an informed decision and knowingly counsel patients that radiologic procedures provide more benefit than harm. Evaluation of a pregnant patient exposed to radiation should involve consideration of the type of radiation, types of examinations performed, gestational age, and radiation dose to determine an estimation of risk. The radiation dose of interest is the dose absorbed by the embryo or fetus and not by the mother. However, recent articles7,8 have raised concern about exposure of maternal breast tissue to radiation and postulate a relationship to breast cancer decades later. The main goals of this chapter are to review the basic issues of pregnancy and radiation exposure and to provide a practical approach for clinicians in choosing a technique that entails the least risk and in counseling patients who have undergone or will undergo an emergency diagnostic procedure. The dose equivalent, expressed in rem or sievert (Sv), is used to quantify the degree of biologic effect (1 Sv = 100 rem). This unit reflects the biologic response and can be used to compare the effects of different types of radiation. The dose equivalent is the product of the absorbed dose times a quality factor. The quality factor depends on the mass and charge of the radiated particle and is approximately 20 for an α-particle and 1 for x-rays and γ-rays. Therefore, for diagnostic radiographs, CT scans, and 99Tc nuclear studies, the absorbed dose is equal to the dose equivalent; that is, an absorbed dose of 1 rad yields a dose equivalent of 1 rem (1 Gy = 1 Sv) (Table 72-1). All reference data have been converted into rad units for uniformity and comparison throughout the chapter. The effects of exposure to radiation on the conceptus depend on the gestational age and the amount of absorbed dose. The relationship between radiation-induced effects and stage of pregnancy is shown in Figure 72-1.9 The harmful effects of ionizing radiation have the following principal biologic effects: intrauterine death, organ malformation, mental impairment, fetal growth retardation, cancer, and genetic mutation.2 Radiation-induced health effects are divided into two broad categories, stochastic and nonstochastic (Table 72-2). Stochastic effects, such as cancer or genetic mutation, can result from alterations produced in a single cell and are presumed to exist even at low exposure.2 The probability of such an effect occurring increases with dose, and there is no identifiable threshold dose below which the chance is known to be zero. It is important to recognize that at low doses of radiation, the risks are far below the spontaneous incidence of carcinogenesis10 or mutagenesis.11 TABLE 72-2 Stochastic and Nonstochastic (Threshold) Comparison Modified from Brent RL. Utilization of developmental basic science principles: the evaluation of reproductive risks from pre- and postconception environmental radiation exposures. Teratology. 1999;54:182. The remaining harmful biologic effects mentioned earlier are nonstochastic effects. Nonstochastic effects require multicellular injury and have a threshold dose below which deleterious effects do not occur.2 It is important to emphasize that the vast majority of embryopathologic effects are believed to be threshold phenomena; therefore, a dose of ionizing radiation below the threshold will not produce these effects. During the preimplantation and implantation phase, the principal radiation-induced health effect is abortion.12 When the number of cells in the conceptus is small and their nature not yet specialized, the effect of damage to these cells is most likely to take the form of failure to implant or undeterminable death of the conceptus.10 The no-effect threshold of an absorbed dose is quite high, estimated at 10 to 15 rad,12 and not likely to be approached by diagnostic ED radiographs or radionuclide testing. By the time that the pregnancy is at term, the threshold for causing intrauterine mortality has risen to about 100 rad.12 These estimates have been extrapolated from animal data. This period has been referred to as the “all-or-none period” because radiation is more likely to kill the embryo than result in a live malformed newborn. Few human epidemiologic data are available for this period of gestation. Because many women are certainly unknowingly exposed to diagnostic radiation during this period, the lack of such data suggests no association between diagnostic radiation exposure and embryo death. Data from the Japanese atomic bomb experience have been cited for reference, but such a correlation is difficult to justify scientifically. Nonetheless, these data show a decrease in the number of offspring who retrospectively would have been 0 to 3 weeks’ postconceptional age at the time of this significant radiation exposure, thus suggesting increased fetal loss caused by irradiation during preimplantation.13 This decrease in the birth rate is probably multifactorial because stress, disease, and malnutrition were coexistent during this traumatic time. Importantly, fetal loss from exposure to an atomic bomb cannot be scientifically extrapolated to exposure to diagnostic radiation. Any discussion concerning the potential adverse effects from diagnostic radiation must take into account the natural incidence of spontaneous abortion. Because the main effect of exposure to radiation during the first few weeks after fertilization is abortion as a result of death of the embryo, it is paramount to note that the normal incidence of spontaneous abortion in humans not exposed to radiation is in the 30% to 50% range.2 Exposure to less than 10 rad yields no statistical change in the rate of preimplantation and early postimplantation spontaneous abortion from the expected baseline (Table 72-3). TABLE 72-3 Risks and Threshold Doses of the Main Effects of Prenatal Irradiation Modified from Fattibene P, Mazzei F, Nuccetelli C, et al. Prenatal exposure to ionizing radiation: sources, effects, and regulatory aspects. Acta Paediatr. 1999;88:693. Exposure to very high-dose radiation, far greater than could be delivered by even aggressive diagnostic radiographs and radionuclide procedures, has been reported to result in teratogenesis. Such information is gleaned from women who were exposed to therapeutic radiation (in the range of 250 rad) during early pregnancy for conditions such as pelvic malignancies. Organ malformations are the main consequence of exposure to radiation during the organogenesis period (3 to 8 weeks). Abnormalities result from killing of cells during the active phase of proliferation and differentiation. Because the embryo is unable to completely replace damaged cells, malformations occur. The most common effects of exposure during organogenesis are malformations in the organs under development at the time of exposure and a reduction in skeletal development. Growth retardation and microcephaly are the predominant effects.10,12,13 These effects have a reported threshold dose range of 5 to 20 rad or higher but are not generally observed unless the exposure is several orders of magnitude or higher.10,12 This dose range is significantly higher than that attained in diagnostic radiology or diagnostic nuclear medicine procedures. Even though ocular abnormalities, developmental facial abnormalities, genital abnormalities, and physical deformities of the extremities have been reported after exposure of the embryo to very high doses, such abnormalities have not been linked to the amount of radiation that would be delivered from even multiple diagnostic radiographic procedures. Importantly, there are no reports of external radiation inducing morphologic malformations in humans unless the offspring also exhibited either growth retardation or a central nervous system (CNS) abnormality. Simply stated, isolated structural abnormalities will not develop in a fetus exposed to radiation. The fear of extra toes, cleft palate, or heart or kidney malformations from exposure to diagnostic radiation during pregnancy is simply unfounded, yet often believed by the general public. Temporary growth retardation is likely with doses in the range of 10 to 25 rad.13 Infants with low birth weight and length may recover fully and attain normal adult stature. The natural incidence of a live birth having a developmental anomaly is 2% to 4%,9 and the incidence of intrauterine growth retardation is 2% to 3%.9 Again, it is important to emphasize that exposure to less than 5 rad yields no change in the risk for occurrence of organ malformations or growth retardation13 (see Table 72-3). Mental Impairment: From the 8th to the 15th week, there is a rapid increase in the number of neurons that migrate to their ultimate sites and lose their capacity to divide. At 15 to 25 weeks, there is more differentiation and architectural definition.9 These embryologic changes make the fetus susceptible to damage to the CNS during the early fetal and midfetal periods. In utero atomic bomb survivor data indicate that the risk for SMR per rad was higher if exposed during the early fetal versus the midfetal period.13 In children exposed to greater than 50 rad between 8 and 15 weeks after conception, a drop in IQ score of 0.3 point per rad was estimated.13 There is no documented increased risk for mental retardation in humans at a gestational age of less than 8 weeks or greater than 25 weeks when evaluated with doses of less than 50 rad.14 The highest risk for SMR occurs during the early fetal period with fetal doses in the range of 100 rad. All clinical observations on significant reductions in IQ and SMR relate to fetal doses of about 50 rad and higher.10 This dose range greatly exceeds the dosages used for diagnostic imaging. It is important to relate the magnitude of radiation effects to abnormalities that occur spontaneously in the population. Multiple causes of mental retardation have been identified, including malnutrition, lead poisoning, rubella infection during pregnancy, and maternal alcoholism. Current prevalence figures indicate that the normal incidence of a person having an IQ below 70 is approximately 3%.10 At fetal doses of 10 rad, the spontaneous incidence of mental retardation is much larger than any potential radiation effect on IQ reduction.10 Regardless of the time of gestation, reduction in IQ cannot be clinically identified with fetal doses of less than 10 rad (see Table 72-3).10 Growth Retardation: The human data for Hiroshima and Nagasaki reveal that the major congenital anomaly observed was microencephaly.13 Studies have not demonstrated any increased risk for microencephaly in the population exposed to less than 150 rad in Nagasaki; however, an increased risk in the Hiroshima population exposed to doses as low as 10 to 19 rad has been reported.14 It is possible that the difference between the two cities is secondary to causes (e.g., trauma, stress, malnutrition) other than radiation. In experimental animal data, a dose of 10 to 20 rad did not increase the incidence of microencephaly.15 The dose threshold for microencephaly, as well as other congenital anomalies, is generally accepted to be in the range of a few rad. Permanent growth retardation is not typically seen unless doses exceed 50 rad.13 Irradiation of the human fetus at doses below 10 rad has not been observed to cause congenital malformations or growth retardation (see Table 72-3).2,4,9 The magnitude of risk for carcinogenesis after low-dose radiation exposure and whether the risk changes throughout gestation have been the subject of many publications,16–18 yet interpretation of the data remains open to date. Numerous studies19–22 indicate a 1.3- to 3.0-fold higher incidence of leukemia in children exposed to diagnostic radiation in utero, although some studies fail to substantiate this association.15,23 Excess cancer as a result of in utero exposure has not been clearly demonstrated in Japanese atomic bomb survivor studies even though the population has been monitored for about 50 years, but the number exposed is not large.10 Identification and control of confounding factors make interpretation of radiation carcinogenesis studies difficult, if not impossible to interpret. Brent and coworkers15 noted that most investigators agree that low doses of radiation present a carcinogenic risk to the embryo; however, findings of increased risk for cancer in children exposed in utero to low-dose diagnostic radiation must be reconciled with the fact that high-dose animal and human studies have not found a marked increase in the incidence of cancer. Risk can be expressed in several ways, including relative risk and absolute risk. In relative risk, the risk is expressed as a function of the “background” risk. For example, a relative risk of 1.0 indicates that there is no effect of irradiation, whereas a relative risk of 1.5 for a given dose indicates that the radiation is associated with a 50% increase in cancer above background rates. The absolute risk estimate simply indicates the excess number of cancer cases expected in a population because of a certain radiation dose.10 The International Commission on Radiological Protection Publication 8410 noted that a recent analysis of many of the epidemiologic studies conducted on prenatal x-ray exposure and childhood cancer are consistent with a relative risk of 1.4 (a 40% increase over the background risk) following a fetal dose of about 1 rad. The best methodologic studies, however, suggest that the risk is probably lower than this. Even if the relative risk were as high as 1.4, the individual probability of childhood cancer after in utero irradiation would be very low (≈0.3% to 0.4%) because the background incidence of childhood cancer is so low (≈0.2% to 0.3%). Absolute risk estimates for cancer from ages 0 to 15 after in utero irradiation have been estimated to be in the range of 600 per 10,000 persons each exposed to 100 rad, or 0.06%/rad.10,12 If a fetus is exposed to 0.1 rad, the increased risk for carcinogenesis is 0.006%, or 3 in 50,000, as compared with the background incidence of 0.2% to 0.3%, or 100 to 150 per 50,000. The increased carcinogenic risk from exposure to 0.1 rad is approximately 50 times smaller than the already low natural incidence of cancer. Investigating possible radiation-induced alterations in the human genome is exceedingly difficult. The geneticists who studied the irradiated populations in Japan are convinced that there were radiation-induced mutations. However, the calculated and confirmed risks were so small that the investigators were unable to demonstrate statistically significant genetic effects.24 The risk for radiation-induced hereditary disease in humans is reported to be around 1% per 100 rad.13,15 If a fetus is exposed to 0.1 rad, the increased risk is approximately 0.001%, or 1 in 100,000. The natural frequency of genetic disease manifesting at birth is approximately 3%,11 or 3000 per 100,000. For 0.1 rad, the increased genetic risk is minute in comparison to the natural incidence of genetic disease. Table 72-4 lists estimated fetal exposure for various diagnostic imaging modalities.25–27 Multiple sources of estimated fetal exposure were reviewed, and the highest reported exposure from these sources is listed. The number of examinations required to reach a cumulative dose of 5 rad is calculated in the second column to underscore the order-of-magnitude difference between the dose considered to have negligible risk (5 rad) and the actual exposed fetal dose. For example, one would require 5000 radiographs of an upper or lower extremity, 12 pelvic radiographs, or more than 5000 two-view chest radiographs before the 5-rad limit is reached. This information is represented graphically in Figure 72-2. TABLE 72-4 Estimated Fetal Exposure from Various Diagnostic Imaging Methods HIDA, hepatobiliary iminodiacetic acid; rad, unit of absorbed radiation. Reproduced from Toppenberg KS, Hill DA, Miller DP. Safety of radiographic imaging during pregnancy. Am Fam Physician. 1999;59:1813. Many variables affect calculation of the fetal radiation dose from CT scans, especially slice thickness, number of cuts, distance of the target organ from the fetus, and gestational age. Table 72-4 summarizes the estimated maximal fetal radiation doses from CT scans. It should be noted that CT of the lumbar spine delivers radiation to the fetus that approaches the safe cutoff range. A CT scan of the abdomen exposes the fetus to less radiation than the 5-rad cutoff, but alternative methods of investigation such as US or MRI should be considered in early pregnancy if the clinical condition warrants. Head CT is the most commonly requested CT scan in pregnancy. The expected fetal absorbed dose is less than 50 millirad (mrad), which is 100 times less than the dose with negligible risk. The estimated radiation dose to the fetus for CT of the chest is less than 0.100 rad. Spiral CT is commonplace in radiology departments and is a popular diagnostic tool used for suspected pulmonary embolism (PE) in pregnant patients. The dose with spiral CT of the chest is less because the duration of the procedure is much shorter.28 Van der Molen29 reported that using 16-slice versus 4-slice CT can equate to a reduction in radiation dose of 20% to 30%. Ordering CT scans of the head, chest, abdomen, and pelvis is a daily occurrence for emergency medicine clinicians. With that in mind, it is sobering to realize that the seventh National Academy of Science report on the Biological Effects of Ionizing Radiation (BEIR VII) indicated that a 10-rad dose is associated with a lifetime attributable risk for development of a solid cancer or leukemia of 1 in 1000.30,31 As data on the effects of ionized radiation accumulate and the technology of nonionizing techniques improves, our use of ionization-based modalities will diminish. The American College of Radiology notes that iodinated low-osmolality contrast media (LOCM), most of which are nonionic agents, have been shown to be associated with less discomfort and a lower incidence of minor (1% versus 5% for high-osmolality contrast media [HOCM]) and severe reactions (0.015% versus 0.1% for HOCM). Many radiology departments routinely use LOCM.32 Although authors have expressed concern over the possibility that iodinated contrast media may suppress fetal or neonatal thyroid function for a short period,7 the added benefit of using nonionic contrast agents is that intravascular use of such agents has been reported to have no effect on neonatal thyroid function.33 Postnatal screening for hypothyroidism is done routinely in the United States. The combination of increased use of iodinated contrast agents in pregnant women to rule out PE and the dearth of literature reporting increased fetal hypothyroidism in the United States supports the report that use of iodinated contrast agents does not affect fetal thyroid function.
Radiation in Pregnancy and Clinical Issues of Radiocontrast Agents
Units of Radiation
Timing of Radiation during Pregnancy and Its Effects
Stages of Fetal Development
Preimplantation and Implantation Phase
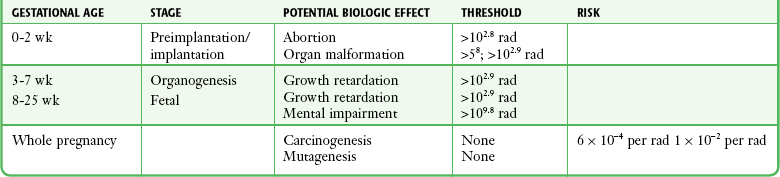
Organogenesis
Fetal Period
Carcinogenesis
Mutagenesis
Radiation Exposure from Diagnostic Radiographs
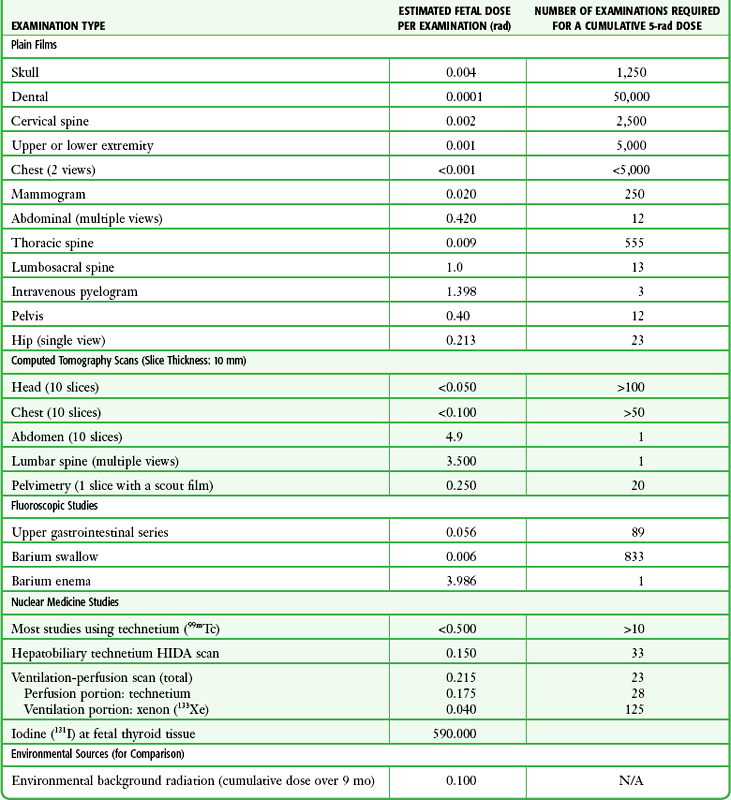
Radiation Exposure from CT Scans
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine