Chapter 48 Rabies Management in Wild Carnivores
Rabies is an acute progressive encephalitis caused by viruses in the family Rhabdoviridae, genus Lyssavirus. This zoonosis is global in distribution, maintained by bats and terrestrial mammalian carnivores. As one of the oldest infectious diseases, control of rabies in free-ranging wild carnivores has run the gamut from ignorance to population reduction prior to the 20th century. In contrast, animal vaccination (both trap, vaccinate, and release [TVR] and oral rabies vaccination [ORV]) has become a novel model for modern interventional wildlife management, using attenuated, modified live or recombinant rabies virus vaccines.5–9,22,24 To put this effect in context, a review has generated an excellent historical perspective of rabies and its control in Europe.10 Given that comprehensive contribution, other very recent literature on the topic, and space and citation limitations, this brief overview attempts to focus largely on timely progress in the field in North America since 2005.16,21
Shift From Population Reduction toward Oral Vaccination
Population reduction and vaccination are based on a similar tenet; that is, the number of susceptible animals is reduced by suppressed population density or by immunization to a threshold at which virus transmission rates are insufficient for rabies to be perpetuated. However, population reduction has proven to be a labor-intensive expensive approach, lacking social appeal as a sustainable control strategy for large land masses. Potential ecologic effects limiting population reduction as a stand-alone approach to rabies control include increased immigration of incubating or rabid animals from surrounding areas in which rabies is enzootic, and enhanced fecundity as a function of suppressed target species densities, leading to relatively rapid (1 to 2 years) repopulation of treatment areas with susceptible animals.13 Whereas successes that may be attributed to population reduction relate to the underlying goals of programs, there is no documentation of elimination of rabies in wild carnivores through population reduction in North America or Europe. Historically, the effect of suppression of fox populations in Europe over many years is reported to have produced only transient lulls in the prevalence of rabies.
More recently, population reduction (live trapping followed by euthanasia) has been integrated on a case by case basis into point infection control (PIC) campaigns conducted to prevent raccoon rabies from becoming established in Canada.13 The PIC tactic calls for population reduction to be applied around detected cases as an initial phase of an integrated emergency rabies control strategy, followed by TVR and ORV, until the goal of disease elimination is achieved. This integrated strategy is credited with success in eliminating raccoon rabies from Ontario and New Brunswick. Quebec is nearing elimination of raccoon rabies using PIC, with only two cases remaining in skunks in the fall of 2009 from initial detection in June 2006 and a peak of 66 cases in 2007. The future role of population reduction in wildlife rabies control will likely be largely reserved for integration into contingency actions on a case-specific basis to enhance ORV or TVR, alone or in tandem, to address local emergencies. Continued objective study is warranted to evaluate the potential contribution of population reduction to other integrated rabies control strategies in specific local settings.
Vaccination: Central Tactic to Rabies Management of Wild Carnivores
Since its inaugural field use, ORV has been widely used in fox rabies control in western Europe, with expansion of control programs into eastern Europe.7,10,12,19,22 In addition to foxes, ORV was expanded to a newly introduced wild carnivore, the raccoon dog (Nyctereutes procyonides) in Finland during 1988. Prior to the first use of a vaccinia-rabies glycoprotein (V-RG) vaccine in Belgium in 1988, all previous ORV programs were conducted with a variety of baits containing vaccine derivatives from a modified live SAD virus strain, including SAD-B19 and SAD-Berne or the newer generation of SAG1 and SAG2 vaccines, which were produced under monoclonal antibody selection that eliminated residual pathogenicity in rodents and other species. Canada followed with ORV in the mid-1980s using ERA vaccine, and has almost achieved elimination of fox rabies virus, once widespread throughout the southern part of the province.15 In 2007, Ontario began experimental field use of a human adenovirus–rabies glycoprotein vaccine (ONRAB) to address residual foci of fox rabies that had spilled over into striped skunks (Mephitis mephitis), and that had also been used in raccoon (Procyon lotor) rabies control.14 The United States was the first country to expand ORV to raccoons in the 1990s, and V-RG use expanded to coyotes (Canis latrans) and unique gray fox (Urocyon cinereoargenteus) variant rabies in Texas.18
Measuring Oral Rabies Vaccination Success
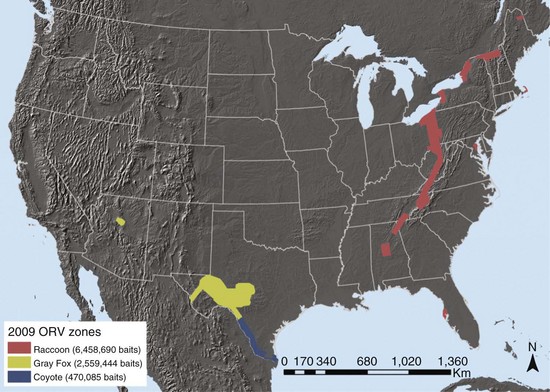
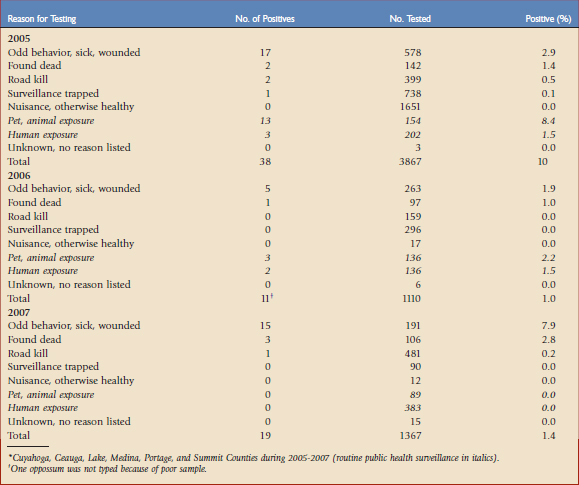
Typically, various ORV components are used as indirect markers of program evaluation, such as bait uptake, biomarker deposition, and seroconversion. Clearly, enhanced rabies surveillance is essential for measuring the most significant progress and success of ORV campaigns related to an actual decrease in disease occurrence.21 The need is perhaps elevated even more when resources limit ORV application to smaller, strategically created vaccination zones near epizootic fronts to prevent spread (e.g., raccoon rabies in the eastern United States with an ORV zone less than 50 km wide). Inherent to enhanced surveillance is increased sampling intensity within and adjacent to ORV zones (Fig. 48-1) to complement information that may result from human and domestic animal exposure events brought to the attention of veterinarians and local public health officials.1 Foci for enhanced surveillance range from road kills to strange-acting—extremely aggressive or docile behavior suggestive of rabies—animals where no human or domestic animal exposure has been reported. Enhanced rabies surveillance within a six-county area encompassing the suburban mosaic east of Cleveland, Ohio, from 2005 to 2007, where a raccoon rabies epizootic began in 2004, illustrates the premium value of testing strange-acting animals not involved in exposure events through enhanced rabies surveillance (Table 48-1). The fact that all rabid wild animal samples were derived from enhanced surveillance sources in 2007 from this area, where exposures had been previously reported annually, provides an ideal example of the value of complementing public health surveillance to make the best informed rabies control decisions. Outreach, local coordination with state, county, and municipal public health officials, state wildlife officials, and veterinarians, and responsiveness in real time to pick up suspect animal samples are some key factors that drive effective enhanced surveillance.
TABLE 48-1 Raccoon Rabies Cases by Sampling Category in an Oral Vaccination Contingency Treatment Area for Ohio*
Access to a direct rapid immunohistochemistry test (dRIT) developed at the CDC has facilitated enhanced local rabies surveillance.1 Under the current paradigm, trained biologists with USDA and Wildlife Services perform the dRIT in several states, allowing for the real-time testing of high numbers of samples without overburdening state rabies laboratories that focus on investigating and diagnosing cases of public health significance as a priority. Since 2005, in excess of 36,000 samples have been tested using the dRIT in 19 states. All positives are confirmed and typed, and all data are entered into RabID, an interactive geographic information system (GIS)–based epidemiologic database with Internet mapping capability to assist in real-time ORV decision making.
Key Oral Rabies Vaccination Program Considerations
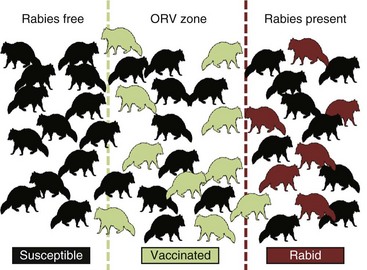
The underlying principle of ORV is that a sufficient proportion of a target population may be vaccinated to function as an immune buffer to disrupt the rabies transmission cycle (Fig. 48-2). As the percentage of the target population immunized increases, the risk of virus transmission decreases as a function of dwindling numbers of susceptible animals in the population. Unlike population reduction, in which removed individuals are ultimately replaced by susceptible animals, ORV may result in the survival of immune individuals for more than 1 year or for the life relatively short-lived (2 to 3 years) carnivores to meet rabies control goals. Oral rabies vaccine efficacy studies are expensive and often tailored to licensing requirements and, as such, often do not evaluate immune effects beyond 6-month study intervals. Retrospective studies of biomarker and virus-neutralizing antibody titers from field vaccinates may be an opportunistic means to evaluate this dynamic.
Vaccine-Bait Biomarker
An effective oral vaccine and bait attractive to the target species is the basic unit in ORV. Ideally, the vaccine and a biomarker are incorporated into the bait matrix, without substantial aversive taste, to ensure consumption and ample vaccine contact in the oral cavity of the target species. Tetracycline remains a commonly used biomarker in oral baits and results in a long-term mark in bone and tooth. Concerns over antibiotic resistance could affect the use of tetracycline for this purpose in the future. A tetracycline biomarker in teeth provides an additional measure of bait uptake by gender and age cohort and is particularly useful if canine teeth are available from harvested animals for evaluation.18 Sampling live-trapped animals for ORV evaluation requires a less intrusive extraction of the first or second premolar tooth, which are less than ideal tissues for consistent tetracycline deposition. The baits commonly used in North America contain the vaccine within a plastic sachet (V-RG) or blister pack (ONRAB) that is covered with the bait or attractants. Generally, bait innovations have not advanced with the pace of generational improvements in oral vaccines and, therefore, remains a critical area for research and development to improve vaccine delivery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree