Chapter 176 Rabbits
Rabbits are popular pets for both children and adults. They are easily litter trained and require minimal maintenance. This chapter stresses diagnosis and management of problems commonly encountered in pet rabbits. Refer to the supplemental readings for more comprehensive information.
BIOLOGIC CHARACTERISTICS
Anatomic and Physiologic Characteristics
Reproductive Characteristics
Normal Parameters
Reference ranges for physiologic values are listed in Table 176-1. Reference ranges for hematologic values, serum biochemical values, and urinalysis are listed in Tables 176-2, 176-3, and 176-4.
Table 176-1 REFERENCE RANGES FOR PHYSIOLOGIC VALUES IN RABBITS
| Temperature | 38–40°C | |
| Heart rate | 130–325 beats/min | |
| Respiratory rate | 32–60/min | |
| Life span | 5–9 yrs | |
| Blood volume | 55–65 ml/kg | |
| Food consumption | 50 g/kg/day | |
| Water consumption | ||
| General population | 50–100 ml/kg/day | |
| Breeding does | <900 ml/kg/day | |
Table 176-2 REFERENCE RANGES FOR HEMATOLOGIC VALUES IN RABBITS
| Erythrocytes | 5.1–7.9 × 106 m3 | |
| Hematocrit | 33%–50% | |
| Hemoglobin | 10.0–17.4 g/dl | |
| Mean corpuscular volume | 57.8–66.5 μm3 | |
| Mean corpuscular hemoglobin | 17.1–23.5pg | |
| Mean corpuscular hemoglobin concentration | 29%–37% | |
| Platelets | 250–650 × 103/mm3 | |
| Leukocytes | 5.2–12.5 × 103/mm3 | |
| Neutrophils | 20%–75% | |
| Lymphocytes | 30%–85% | |
| Monocytes | 1%–4% | |
| Eosinophils | 1%–4% | |
| Basophils | 1%–7% | |
Table 176-3 REFERENCE RANGES FOR SERUM BIOCHEMICAL VALUES IN RABBITS
| Albumin | 2.4–4.6 g/dl |
|---|---|
| Alkaline phosphatase | 4–16 U/L |
| Amylase | 166.5–314.5 U/L |
| Bicarbonate | 16–38 mEq/L |
| Blood urea nitrogen | 13–29 mg/dl |
| Calcium | 5.6–12.5 mg/dl |
| Chloride | 92–112 mEq/L |
| Cholesterol | 10–80 mg/dl |
| Creatinine | 0.5–2.5 mg/dl |
| Globulin | 1.5–2.8 g/dl |
| Glucose | 75–155 g/dl |
| Glutamic-oxaloacetic transaminase | 14–113 U/L |
| Glutamic pyruvate transaminase | 48–80 U/L |
| Lactic dehydrogenase | 34–129 U/L |
| Phosphorus | 4.0–6.9 mg/dl |
| Potassium | 3.6–6.9 mEq/L |
| Serum protein | 5.4–8.3 g/dl |
| Sodium | 131–155 mEq/L |
| Total bilirubin | 0.0–0.7 mg/dl |
| Total lipids | 243–390 mg/dl |
Table 176-4 REFERENCE RANGES FOR URINALYSIS IN RABBITS
| Urine volume | ||
| Large breeds | 20–350 ml/kg/day | |
| Average breeds | 130 ml/kg/day | |
| Specific gravity | 1.003–1.036 | |
| Average pH | 8.2 | |
| Crystals present | Ammonium magnesium phosphate, calcium carbonate monohydrate, anhydrous calcium carbonate | |
| Casts, epithelial cells, or bacteria present | Absent to rare | |
| Leukocytes or erythrocytes present | Occasional | |
| Albumin present | Occasional in young rabbits | |
PATIENT MANAGEMENT
Caging
Diet
Clinical Techniques
Restraint

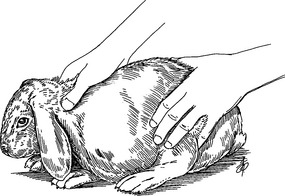
Figure 176-1 To examine the ventrum and anal area, cradle the rabbit as shown. Be sure to provide support to the hind limbs.
Diagnostic Techniques
Venipuncture
Radiography
Treatment Techniques
Table 176-5 DRUGS COMMONLY USED IN RABBITS
| Drug | Dose | Comments |
|---|---|---|
| Antimicrobials/Antifungals | ||
| Benzathine, penicillin G | 42,000–84,000 IU/kg q7d × 3 treatments SC | For treatment of Treponema cuniculi |
| Chloramphenicol | 30–50 mg/kg q12h PO | |
| Ciprofloxacin | 10–20 mg/kg q12–24h PO | Have a suspension made by a compounding pharmacist for easy administration |
| Enrofloxacin | 5–15 mg/kg q12h PO, SC, IM | Limit subcutaneous and intramuscular administration due to potential tissue necrosis at injection sites |
| Gentamicin | 4 mg/kg q24h IM, IV, SC | Use with caution or avoid use |
| Griseofulvin | 12.5 mg/kgq12h PO | |
| Penicillin | 40,000–60,000 IU/kg q48hr SC | Use with caution |
| Tetracycline | 50 mg/kg q8–12h PO | |
| Trimethoprim/sulfa | 30 mg/kg q12h PO, IM, SC | |
| Antiparasitics/Insecticides | ||
| Fenbendazole | 10–20 mg/kg PO, repeat in 14d | |
| Lime sulfur solution | 2.5% dip q7d for 4 weeks | Used in young animals for treatment of mites, fleas, fungal dermatitis |
| Ivermectin | 0.2–0.4 mg/kg q10–14d SC for 2–3 treatments | Effective against ear and fur mites |
| Piperazine citrate | 200 mg/kg; repeat in 2 weeks | |
| Pyrantel pamoate | 5–10 mg/kg; repeat in 2 weeks | |
| Pyrethrin products | Topically as directed q7d | |
| Selamectin | 6 mg/kg topically | |
| Sulfadimethoxine | 50 mg/kg PO first dose, then 25 mg/kg q24h PO for 10–20 days | For treatment of coccidiosis |
| Tranquilizers/Premedications | ||
| Acepromazine | 0.5–1.0 mg/kg IM/SC | |
| Atipamazole | Give same volume SC as medetomidine | Reversal for medetomidine |
| Diazepam | 1–3 mg/kg IV, IM | Used in combination with ketamine |
| Glycopyrrolate | 0.01–0.02 SC | |
| Ketamine | 20–50 mg/kg IM | |
| Ketamine/acepromazine | 40 mg/kg (K)/0.5–1.0 mg/kg (A) IM | |
| Ketamine/diazepam | 10–15 mg/kg (K)/0.3–0.5 mg/kg (D) IM, IV | |
| Ketamine/medetomidine | 0.15–0.35 mg/kg (M) IM/5–20 mg/kg (K) IV later | |
| Ketamine/midazolam | 25 mg/kg (K)/≤ 2 mg/kg (M) IM | |
| Medetomidine | 0.25 mg/kg IM | |
| Midazolam | 1–2 mg/kg IM or slow IV | |
| Propofol | 2–15 mg/kg IV | |
| Xylazine | 1–5 mg/kg SC, IM | |
| Analgesics | ||
| Aspirin | 10–100 mg/kg q8–24h PO | |
| Buprenorphine | 0.01–0.05 mg/kg q6–12h SC, IM, IV | |
| Butorphanol | 0.1–1.0 mg/kg q4–6h SC, IM, IV | |
| Carprofen | 1.0–2.2 mg/kg q12h PO, SC, IM | |
| Flunixin meglumine | 1.1 mg/kg q12–24h SC, IM | |
| Ibuprofen | 2.0–7.5 mg/kg; PO q12–24h | |
| Ketoprofen | 1 mg/kg q12–24h IM | |
| Morphine | 2–5 mg/kg q2–4h SC, IM | |
| Oxymorphone | 0.05–0.20 mg/kgq8–12h SC, IM | |
Tranquilization and Anesthesia
DERMATOLOGIC PROBLEMS
Dermatitis/Alopecia
Etiology
Clinical Signs
Diagnosis
Determine the primary cause of the alopecia to make a diagnosis.
Ear Mites (Psoroptes cuniculi)
Etiology
Clinical Signs
Treatment
Fur and Mange Mites
Etiology
Clinical Signs
Diagnosis
Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree