Rabbit Basic Science
1.1 Biological characteristics of the domestic rabbit (Oryctolagus cuniculus)
1.1.1 Origins of the domestic rabbit
Domestic rabbits are descended from the European rabbit, Oryctolagus cuniculus. The ancestral form probably evolved in the Iberian Peninsula and spread to other parts of the Mediterranean (Fox, 1974). Fossil records show that the European rabbit was confined to the Iberian peninsula and southern France following the Pleistocene era. While rabbits have been associated with man since Roman times, they have only been truly domesticated for around 200 years. The geographical range of the rabbit has been altered significantly by man, who from Roman times onwards placed rabbits on islands on various shipping routes, to allow them to breed and form a ready source of food. Thus their European range significantly altered, and rabbits proved very successful where the climate and geographical conditions were suitable. More recently rabbits were introduced in Victoria, Australia, where their success, due to rapid breeding and a lack of suitable predators, rapidly became a plague that cost the Australian Government millions of dollars and led to the implementation of myxomatosis virus for biological control. Although European rabbits have been released in North America, the presence of suitable predators, an unsuitable climate and other species filling their ecological niche proved insurmountable. In North America the native wild rabbit is either Sylvilagus floridanus (cottontail) or Sylvilagus bachmani (brush rabbit). The North American jackrabbit, Lepus californicus, is from the hare genus.
It is not clear when the European rabbit was introduced into Great Britain. The Romans brought many food animals with them, such as pheasant and quail, and it is believed that they not only introduced rabbits but also kept them in cages, thereby starting the process of domestication. The modern pet rabbit still retains many of the characteristics of its wild counterparts despite changes in size, colour, coat texture and temperament.
Rabbits belong to the order Lagomorpha, which are characterized by the presence of a second small pair of upper incisors or peg teeth situated behind the central incisors. Lagomorphs were once considered to be a suborder of the Rodentia, which is divided into Sciuromorpha (squirrel-like rodents), Myomorpha (mouse-like rodents) and Hystricomorpha (porcupine-like rodents) that includes guinea pigs and chinchillas. Current opinion suggests that Rodentia and Lagomorpha have no fundamental similarities and on the basis of structural features and serological data, Lagomorpha show more affinity to Artiodactyla (hoofed mammals) (Nowak, 1999). Other lagomorphs include hares and pikas. All members of the Lagomorpha order are terrestrial and eat only vegetation.
1.1.2 Wild rabbits
The behavioural characteristics of lagomorphs differ between species. For example, cottontails (Sylvilagus spp.) do not dig burrows, although they may use burrows made by other animals. Females dig holes to make nests and sit over the hole to suckle the young. Vegetation is used to cover the fur-lined nest between feeds. Cottontails are solitary animals, in contrast with Oryctolagus cuniculus, which live in groups with a defined social hierarchy (Nowak, 1999).
The European rabbit, Oryctolagus cuniculus, prefers a sandy, hilly terrain with shrubs and woody plants and is not found at altitudes above 600 m. It often digs complex burrows or warrens that can be 3 m deep and 45 m long. The tunnels are about 15 cm in diameter and the living chambers 30–60 cm high. The main surface entrances are usually indicated by mounds of earth but there are numerous other small openings that lack these mounds. Oryctolagus cuniculus is essentially nocturnal, leaving the burrow in the early evening and returning in the morning, although it can be seen grazing or basking during the day. The home range is rarely larger than 20 hectares (Nowak, 1999).
Wild rabbits live in groups of two to eight adults plus juveniles with a defined social hierarchy (McBride, 1988). The group’s territory is defended by the males, while the females dig out deep burrows to nest in. Male rabbits within the group will establish a dominance hierarchy, with the older heavier males at the top. Aged males that have been usurped by younger, fitter rabbits are driven from the group to become solitary satellite males (Lockley, 1978). Young male rabbits are also often driven from the group when they reach puberty either to join another warren or to lead solitary lives in the hedgerows. The females tend to remain within the original group. Female rabbits are less aggressive towards each other than males, but will defend a chosen nesting site ferociously. Territories are scent marked with pheromones from the scent glands on the chin and genital area or by urine marking. Dominant males will continually scent mark their territory by rubbing their chins on branches and bushes and leaving piles of strategically placed faeces. They also mark territory by spraying urine, sometimes on to other individuals.
When wild rabbits emerge from their burrows at dusk, they begin to feed. Initially, they graze grass and vegetation, raising their heads at intervals to survey the surroundings, perhaps chewing through a long stalk or blade of grass at the same time. After half an hour or so, they will start to look around for other palatable plants to nibble. They are constantly on the lookout for danger and will readily bolt back to their burrow. Hard faecal pellets are always voided above ground, never in the burrow and soft caecotrophs are usually consumed during periods of rest underground, although occasionally rabbits exhibit this behaviour above ground (Lockley, 1978). The only vocal sounds that are made are a loud high-pitched scream of terror or a range of growls and hums that denote pleasure or defence. Apprehensive or frightened rabbits will thump the ground with their hind feet. The loud thumping sounds acts as an alarm signal to other rabbits in the vicinity.
Many of the behavioural characteristics of their wild ancestors are still present in the modern-day pet rabbit. Domestication has resulted in rabbits that are far tamer than their wild counterparts and easy to handle. Although some domestic rabbits still retain the tendency to dig holes and live underground, many do not, with the result that domestic rabbits that escape or are released do not survive for long in the wild. Conversely, wild rabbits seldom become tame in captivity, although the occasional individual will overcome its fear of humans. Hand-reared orphans usually grow into fearful adults. Even rabbits that are born as a result of egg transfer from a wild rabbit to a domesticated tame host retain their shy nature (Adams, 1987).
1.1.3 Breeds of rabbits
Domestication has resulted in a wide range of breeds with different attributes. They can be roughly divided into two groups: fancy breeds and fur breeds (Sandford, 1996). The fur breeds include rex, Angoras and satin rabbits with their beautiful coat textures. Fancy breeds include the Belgian hare, and lop and dwarf rabbits with their varying physical characteristics. Most pet rabbits belong to the smaller breeds such as dwarf lops, Dutch or English. Pedigree rabbit breeders often sell surplus stock to the pet trade and occasionally one of the more obscure breeds may turn up as a pet. Pedigree stock is identified by aluminium rings slipped over the hock when the rabbit is 8–10 weeks old. The rings are supplied by the British Rabbit Council in a range of sizes. Each ring has the year of birth and a unique number from which the rabbit can be identified. Many pet rabbits are the result of interbreeding between pets and are cross-breeds. As with other domestic animals, there are breed predispositions to disease. For example, dwarf rabbits are prone to congenital incisor malocclusion (Fox and Crary, 1971). Dutch, Havana and tan rabbits have a high incidence of uterine neoplasia (Greene, 1941).
1.1.4 Angoras
Angoras have been bred for wool production for hundreds of years. The wool is plucked or sheared and either spun on its own or mixed with sheep’s wool. Plucked wool is superior to shorn wool. Commercial Angoras are kept in a specialized manner to prevent staining and matting of the fur. After defleecing, woollen jackets can be worn for 2–3 weeks to reduce heat loss or a strip of fleece can be left along the back (Lebas et al., 1998). Commercial Angoras are not provided with bedding but are kept on wire mesh floors and hay is provided in a rack. The long fine coat is a definite disadvantage for the pet animal as it difficult to keep the rabbit free from knots and mats. It is not surprising that a high number of Angoras arrive at rescue shelters for rehoming. The breed is prone to intestinal obstruction by felts of ingested hair.
1.1.5 Diurnal rhythms
Many behavioural and physiological processes of rabbits show a marked diurnal rhythm. In the late afternoon wild rabbits emerge from their burrows to feed, explore, socialize and mate. Grazing resumes during the early morning before the rabbit returns to the warren. Hard faecal pellets are voided during these periods above ground. During the day, caecotrophy (see Section 1.3.1) takes place while the rabbit is resting in the burrow, typically between 08.00 and 17.00 h. Female rabbits give birth in the morning and feed their young at night (McBride, 1988). Domesticated rabbits also follow a natural daily rhythm. Laboratory rabbits that are fed ad lib consume little food between 06.00 h and midday and increase their intake between 17.00 h and midnight, eating most food during the night. Caecotrophs are expelled during periods of minimal feed intake in the morning and sometimes during the evening. If food is restricted, caecotrophs are excreted approximately 5 h after a meal. If a collar is fitted to prevent the ingestion of caecotrophs, feeding still ceases during caecotroph excretion, suggesting that cessation of food intake is not associated with gastric filling (Hörnicke et al., 1984).
The diurnal feeding pattern affects digestive processes and caecal motility, which also follow a circadian rhythm. Ingestion of food is associated with increased caecal motility and the excretion of hard faeces. Caecotrophy is associated with a decline in caecal contractions, so caecal contractions are at a maximum when the animal is feeding. If food is withheld completely, the circadian rhythm of caecal contractions is maintained, but at a lower frequency that does not correlate with soft or hard faeces production (Hörnicke et al., 1984). Absorption of volatile fatty acids and their metabolism in the liver shows a circadian rhythm parallel to the activity of the adrenal gland. Volatile fatty acid absorption into the portal circulation is greatest during the hard faeces phase of digestion, although arterial levels remain remarkably constant (Vernay, 1987). Bile acid production shows a circadian rhythm (Fekete, 1989). There is a diurnal variation in haematological values (Fox and Laird, 1970), with lowest total white blood cell and lymphocyte counts occurring in the late afternoon and evening in association with increased neutrophil counts. Eosinophil counts peak during the afternoon, with the lowest values occurring in the morning. Blood urea nitrogen shows a diurnal variation that is linked with feeding patterns. Even body temperature follows a 24-h cycle (Lazarus-Barlow, 1928).
1.2 Housing and husbandry
1.2.1 Housing
The quiet docile nature of the rabbit combined with its fertility and rapid growth rate has led to its intensive production for commercial and laboratory purposes. Units housing several thousand does are found in countries such as China, Hungary and the USA. At the other end of the scale, in the developing world, a few rabbits are often kept as ‘biological refrigerators’, i.e. a source of small quantities of meat that is fresh and readily available and which can be eaten before it goes off (Cheeke et al., 1982). The social and behavioural needs of such animals are ignored when they are housed individually in small, wire mesh cages or confined to tiny hutches. The Royal Society for the Prevention of Cruelty to Animals (RSPCA), the Department for Environment, Food and Rural Affairs (DEFRA) and other animal health and welfare associations promote the ‘Five Freedoms’ of animal welfare:
in order that guidelines for animal husbandry and nutrition as far as possible advance the welfare of pet and production animals. There are many welfare implications associated with keeping rabbits in cages, as they are not able to follow their natural instincts.Abnormal behaviour patterns such as stereotypies and restlessness have been recorded. Perpetual wire biting and pawing behaviour has been described in rabbits confined to small cages and does provided with an open nesting box and no bedding material to cover the young (Stauffacher, 1992). A proven link has been established between small cage size and painful conditions such as skeletal disorders or ulcerative pododermatitis in intensively reared rabbits (Drescher, 1993; Drescher and Loeffler, 1996). Morphological differences have been observed in the adrenal glands of rabbits kept in wire cages and those kept in group housing conditions on solid floors (Drescher and Breig, 1993).
In recent years, conditions have improved for many laboratory rabbits. They can be kept in social groups of four to eight animals with no detriment to their health (Turner et al., 1997). It has been proven that rabbits prefer to be in proximity with each other as they are a social species with a defined hierarchy. They also ‘interact with enrichment objects’ such as wooden sticks, parrot toys or balls designed for cats (Huls et al., 1991). Keeping rabbits in this way not only benefits the rabbits but also the people looking after them. Love (1994) described the response of animal technicians to group housing by saying they ‘found it more agreeable to work with rabbits that came to the front of the cage when they heard the sounds of people, rather than cowering away’ and ‘it was a pleasure to see the rabbits interact with each other’. Stauffacher (1992) describes in detail many ways in which housing for rabbits can be constructed to permit natural behaviour patterns. Despite these advances, most breeding and exhibition rabbits still live their entire life confined to small cages. Some breeders still insist that rabbits should be kept singly in small cages and that large hutches and runs lead to aggression and behaviour problems (Sandford, 1996). At last, the pet-owning fraternity is becoming aware of the rabbit’s social nature and need for exercise. There has been a steady increase in the number of house rabbits and the status of the rabbit has shifted from the child’s pet to a member of the family. A rabbit can be a satisfactory companion for adults that are out at work all day and find the needs of a dog or cat too demanding. Hopefully the days of keeping pet rabbits in solitary confinement in a barren hutch at the bottom of the garden are now coming to an end. There is legislation governing the welfare of rabbits that is summarized in Box 1.1.
1.2.2 Hutches for pet rabbits
Traditionally, pet rabbits are kept in hutches in the garden, shed or garage. Hutches are a convenient method of housing rabbits; however, most hutches on sale today do not provide sufficient space for rabbits to display their natural behaviours. It is important to provide time outside their hutch for exercise each day. At least 4 h daily exercise is required (Richardson, 2000a). Longer periods or unrestricted exercise are preferable.
The hutch should be as big as possible, especially if two rabbits are housed together. It needs to be situated in a dry, cool, well-ventilated site protected from wind and rain. The minimum recommended size is sufficient space to hop three times in any direction, and high enough for the rabbit to stand up on its hind legs without its ears touching the roof. This space should be available in both the dark, covered portion of the hutch and the open living space. This space requirement should not include the outdoor run. The RSPCA spatial requirements are somewhat greater, the minimum being 1 m (3 ft) wide × 2 m (6 ft) long × 1 m (3 ft) high for both the enclosed and open portions of the living area. In any case where two adult rabbits live together, the space must be increased proportionally and suitable hide areas provided so that each rabbit can get away from the other if they want. The optimum temperature range for rabbits is 15–20°C, which can be checked with a maximum and minimum thermometer. Above this temperature rabbits can suffer from heat stress. Poor ventilation and ammonia build-up predispose to conjunctivitis and respiratory tract infections. It is preferable to situate the hutch against a sheltered wall outside, rather than in an enclosed garage with potential exposure to toxic car fumes. Placing the hutch in a large airy shed can be a good option as shelter is provided with the option of protected exercise space. Many rabbit owners are now using a whole garden shed as a kind of ‘super-hutch’ for housing two or more rabbits, providing ample indoor space. These sheds are then often attached to large aviary type runs to allow outside access (See Figure 1.1). Rabbits are tolerant of cold conditions and can withstand winter weather provided they have shelter and plenty of bedding material. Thin rabbits with no body fat are more susceptible to the effects of cold and need extra protection on cold nights. Hot conditions and direct sunlight with no shade are distressing for rabbits. These conditions are potentially fatal as rabbits cannot sweat or pant effectively, and are far more damaging than cold conditions. The ability to get out of direct sunlight is of paramount importance, and needs to be considered when providing rabbit accommodation. Rabbits are far more capable of coping with cold and even wet conditions, as long as they have the opportunity to shelter and access to enough food and bedding. When planning rabbit accommodation, allowing the rabbit a choice of positions and therefore ambient temperatures, access to sunlight and shelter is a major benefit. Rabbits produce copious quantities of urine and faeces, which are usually deposited in one part of the hutch that should be cleaned once or twice daily. Bedding that is not fouled and remains clean and dry can be left in the hutch; however, with any deep litter system, a full clean should be done at least weekly, or more often if the bedding is wet or smells. Many types of material can be used as bedding. Any bedding material should be non-toxic, free from dust, comfortable to lie on and good insulation. Garden peat has been recommended to neutralize ammonia and reduce irritation to the eyes and respiratory tract (Malley, 1995). An economical bedding material is a layer of newspaper covered in hay. This can be rolled up for disposal. The hay provides ad lib high fibre food in addition to a soft bed that is kind to the hocks. Other options include straw of various types. The advantage to straw is that it is cheap, easily available and very good at allowing fluid to drain away from the surface of the bedding, reducing potential contact with rabbit skin. Straw can be fairly sharp, depending on what type of crop it is made from, and can in some circumstances cause skin and ocular irritation. Rabbits will sometimes eat straw, which can cause oral trauma if it is sharp. Oat straw is an ideal option as it is soft as well as encouraging fluid drainage. Commercial forms of bedding are also available. These are often recycled paper products, and are well accepted by rabbits, although they are not the most economical bedding. Woodshavings and chips are not recommended because of the potential for dust to cause ocular and respiratory irritation and the possibility of sharp edges causing skin/mouth/eye wounds. Wood products containing aromatic oils may cause respiratory irritation and have been reported to cause hepatotoxicity.
1.2.3 Exercise
Exercise is of paramount importance for the physical and mental health of rabbits. Immobile rabbits are at increased risk of ulcerative pododermatitis, osteoporosis, urine sludging and spinal fractures. There is a proven association between confinement and the development of spinal deformities (Drescher and Loeffler, 1996). Exercise improves blood circulation and prevents pressure sores. The opportunity to explore is mentally beneficial. All methods of providing exercise should be escape proof, although escapees instinctively remain close to their home territory and can usually be found providing they have not been carried off by a predator or are in search of a receptive mate. Any outside exercise area should be as large as possible and allow the rabbit sufficient space to run rather than just hop.
Grass and natural vegetation is the ideal diet for rabbits. Access to a garden, enclosure or pen outside provides nutrition as well as environmental enrichment. Natural daylight is the best way of providing the correct amount of vitamin D for the animal’s needs. Rabbits enjoy basking in the sun. However, their destructive and burrowing instincts, along with a taste for bedding plants, means free access to the garden should be supervised. Free rabbits are also prey to neighbours’ dogs, cats and other animals such as foxes, so supervision or a well-fenced area or mesh pen is required. Ideally this should be a permanent structure that allows the rabbit to establish a familiar territory and feel secure. An area of approximately 3 m2 (10 sq. ft) is sufficient, although larger areas are preferable. Branches, drainpipes, boxes and other enrichment objects can be placed in the enclosure to provide cover and recreation. Planting of suitable weeds/plants inside the enclosure is also a good idea.
It is possible to train rabbits to return to their hutch at specified times of the day by rewarding them with food. Many pet rabbits are tame enough to be picked up, especially if they have been handled daily from an early age. These animals can be given free access to a garden during the day, perhaps under supervision, and returned to the hutch at night. Alternatively, portable wire mesh runs can be used, the familiar territory being sacrificed for the ability to provide a fresh area of forage regularly. There are many designs, some of which can be moved around the lawn, allowing the rabbits to keep the grass down. Enclosed yards are an acceptable alternative to a garden. Rabbits can also be allowed to exercise in the house. In either situation tomato trays planted with edible vegetation (seed packages of suitable plants are available) can provide environmental enrichment.
1.2.4 Burrowing
The opportunity to dig their own burrow is appreciated by many rabbits but not by their owners. Once they have dug out and established a burrow, most rabbits appear satisfied and do not start another. Females are more likely to dig burrows than males as their instinct is to dig out new nesting sites, especially during the spring. Pregnant or pseudopregnant does exhibit marked burrowing behaviour, although it can still be exhibited by spayed females. Burrowing can be accommodated in an outside run with a little imagination, attention to escape potential and buried wire walls. Despite the potential inconvenience, allowing rabbits to perform natural behaviours such as burrowing can make a significant difference to perceived well-being in pet rabbits.
1.2.5 Companionship
Rabbits are social creatures that benefit from companionship, preferably from another rabbit. A bonded pair becomes inseparable (see Figure 1.2). They spend time grooming each other and there are many benefits to mutual grooming, such as reducing parasite numbers in the fur and cleaning inaccessible places such as the face or back of the neck. Occasionally, a dominant rabbit will barber the fur of its companion. Neutering rabbits that are kept together is necessary to prevent fighting and unwanted pregnancies. Guinea pigs are sometimes kept as companions for rabbits, although this arrangement is not as satisfactory as two rabbits together. There is a small risk of the guinea pig contracting Bordetella bronchiseptica, which is asymptomatic in rabbits but can cause pneumonia in guinea pigs. Neutering is required to prevent bullying and constant sexual harassment of the guinea pig. Regardless, some rabbits will still bully their guinea pig companion, and hide boxes small enough to allow the pig in but exclude the rabbit should be provided. If the bullying is severe the welfare of the guinea pig is better served by breaking up the pair. Choosing to pair a rabbit with a guinea pig should not be advocated; however, where a stable pair exists, then the bond is similar to that between two rabbits and should not be broken unless the welfare of the guinea pig is suffering.
Rabbits have distinctive personalities and strong individual likes and dislikes of other rabbits. It is not possible to predict accurately whether newly introduced rabbits will form an instant rapport or attack each other. Neutered rabbits of the opposite sex are most likely to bond, although it is possible to keep same sex pairs together. Pairs of male rabbits need to be castrated to prevent fighting. Ideally, rabbits should be introduced on neutral territory with plenty of room for escape and hiding places to retreat into. If this is not possible, introducing the female to the male on his territory is more likely to be successful than the reverse. When they are first introduced, most rabbits spend a period chasing each other around and pulling some hair out, but will settle down eventually. A rabbit that has spent its entire life confined to a hutch may not realize that it can run around and is daunted by both the great outdoors and its new companion. It is not unusual for such rabbits to remain quiet and immobile for several days before they gain confidence and start to explore. If possible, a period of separated proximity is advisable to allow rabbits to become accustomed to each other’s presence before they are introduced. Adjacent pens separated by wire mesh allow rabbits to sniff each other; also change around the bedding to get the rabbits used to the scent of the proposed companion in their own territory. It is a promising sign when the two are found lying side by side on either side of the mesh. Some rabbits never bond; others accept any new companion readily.
1.2.6 Winter housing
The advice is often given that rabbits should be given shelter from the winter weather by bringing the hutch into a shed or garage. As a result, many rabbits do not come out of their hutch for 6 months of the year because owners fear their pet will ‘catch a chill’. It is important to provide exercise during the winter as well as the summer. Free-range rabbits that are kept outside all year round often choose to sit in the rain and snow despite having a hutch full of warm bedding to go into if required. They seem impervious to cold and as long as they have access to shelter, plenty of food and protection from predators, rabbits can be kept outside during the winter. Thin or ill rabbits or those that have not been acclimatized should not be kept in this fashion and need to be given extra protection indoors or in a hutch or shed during inclement weather. They can be allowed outside if the weather is good. If rabbits are not exposed to natural daylight during the winter months, vitamin D deficiency can occur. Undetectable vitamin D levels have been found in blood samples taken in spring from pet rabbits housed in hutches over winter (see Figure 1.5) (Fairham and Harcourt-Brown, 1999).
1.2.7 Free-range rabbits
Stauffacher (1992) described the behaviour of rabbits under ‘near-to-nature’ or free-range conditions. The rabbits were kept in an open-air turfed enclosure with several trees and bushes. They were kept in groups of up to 30 animals and their daily activities followed a double diurnal rhythm with periods of rest alternating with periods of activity around dusk and dawn. During periods of rest, the rabbits sought out places with a good overview of the enclosure under bushes or near trees where they would huddle together and engage in mutual grooming. This method of husbandry permits natural behaviour patterns, encourages grazing and normal caecotrophy and allows animals to groom themselves and each other thoroughly, thereby removing skin debris, dead hair and parasites from the coat. In a study by Harcourt-Brown and Baker (2001) blood samples from rabbits kept under free-range conditions had higher red cell and lymphocyte counts than rabbits kept in hutches, suggesting that they were healthier (see Figure 2.1).
1.2.8 House rabbits
In recent years, there has been a trend to give pet rabbits the run of the house. House rabbits make good companions and can be trained to use a litter tray. They are usually provided with some sort of sleeping accommodation to which they can retreat and can be confined while their owners are out at work. Many house rabbits have their own room that contains an open hutch or childs ‘wendy-house’ for sleeping. Most house rabbits are neutered, especially males, to reduce territory marking by spraying or defecating outside the litter tray. Rabbits can bond closely with human owners and make entertaining responsive pets. They will play with toys, beg for treats and follow their human companion around the house. Dogs and cats can learn to tolerate rabbits as companions. Rabbits can also learn not to view dogs and cats as predators.
1.2.9 Litter trays
Large cat litter trays or gravel trays from the garden centre can be used for rabbits to urinate and defecate in. Hay, straw, cat litter, peat, soil or ‘natural’ litters made from hemp, corn cobs or reclaimed wood pulp are all used as litter, materials for rabbits. Clay litters are not advisable as some rabbits will eat the litter, which can then impact the caecum (Brown, 1997).
Organic solvents in litter materials derived from preserved pine wood shavings or cedar chips have been reported to cause hepatotoxicity and are therefore inadvisable (Rabbit Health News, 1991b). Hay or clean, chopped straw can be used in rabbit litter trays.
1.2.10 Thermoregulation
Rabbits are unable to sweat or pant effectively to dissipate body heat. The main thermoregulatory mechanism is by heat exchange in the ears, which have a large arteriovenous anastomotic system. In the nose, the nasal glands moisten inspired air, which also has a role in thermoregulation. Rabbits are unable to tolerate high ambient temperatures, which can prove fatal.
1.3 Digestive anatomy, physiology and nutrition
1.3.1 Digestive physiology
The alimentary tract of the rabbit is adapted for the digestion of large quantities of fibrous food (Figure 1.3). Rabbits have developed a strategy of high feed intake and rapid food transit through the gut to meet their nutritional needs from a nutrient dilute diet. Rabbits are hindgut fermenters and rely on microbial fermentation of food within the caecum to provide nutrients. In the stomach and small intestine, digestion and absorption of nutrients is similar to monogastric mammals. The end-products of the digestive processes are separated in the colon into indigestible material and substances that can be metabolized by caecal micro-organisms. Separation of the ingesta depends on particle size. The proximal colon of the rabbit is specially adapted for the separation of large particles of indigestible fibre from smaller particles that can be degraded and used as a substrate for bacterial fermentation in the caecum. The two components are simultaneously sent in opposite directions.
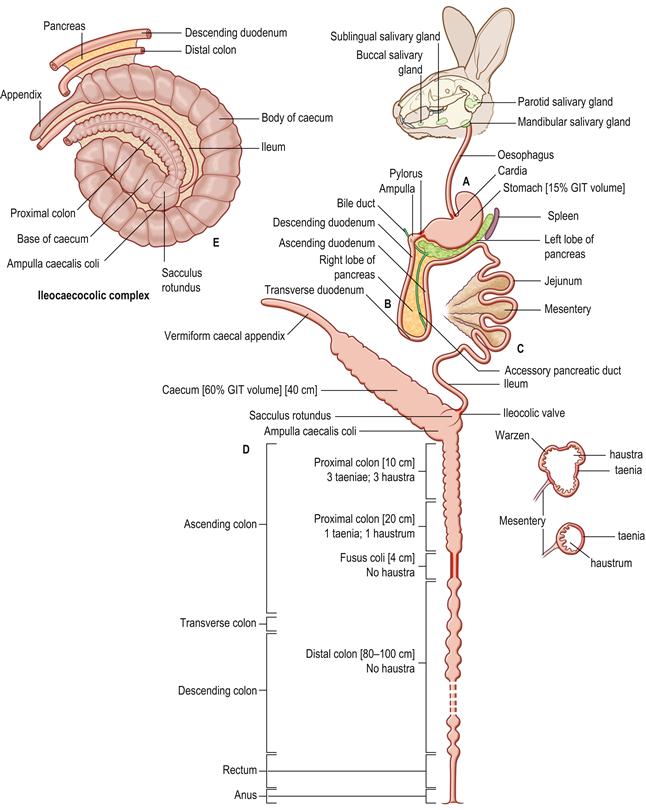
Figure 1.3 Schematic diagram of the anatomy of the alimentary tract of the rabbit.
(A) The alimentary tract of the rabbit is adapted for the digestion of large quantities of fibrous food. The teeth continually grow and wear against each other to maintain their shape. The incisors are worn to a fine cutting edge that can be used to slice through vegetation or gnaw hard substances such as bark or wood. The occlusal surfaces of the cheek teeth are worn to an effective grinding surface that is used to reduce food particles to a small enough size to be swallowed. There are a number of well-developed salivary glands. The cardia and pyloric sphincter are muscular and well-developed. The relatively voluminous stomach is simple in type and always contains food. The stomach contents comprise approximately 15% of the contents of the gastrointestinal tract. (B) The duodenum forms a loop with descending, transverse and ascending parts. It has an extensive mesentery. The duodenum begins with a slight enlargement approximately 1 cm from the pylorus that receives the bile duct. The right lobe of the pancreas is widely dispersed in the mesoduodenum as many isolated lobules. The main body and left lobe of the pancreas run in the mesentery that attaches the transverse colon to the stomach and spleen (see Figure 8.2). A single accessory pancreatic duct opens into the junction between the descending and transverse duodenum. (C) The jejunum is long, convoluted and relatively free of attachments. It occupies the dorsal half of the left flank and the caudal half of the abdomen (see Figures 1.13–1.15). The ileum is closely associated with the mesentery that connects part of the ascending colon to the caecum to form the ileocaecocolic complex (see E). The end of the ileum is expanded into a thick-walled sacculus rotundus. (D) The caecum and appendix are shown as a straight tube, but are in fact a coiled spiral (see E). The thin-walled caecum is a large organ that ends in an appendix that is heavily endowed with lymphoid tissue. The ascending colon of the rabbit can be divided into four sections. The first section is approximately 10 cm long and has three longitudinal flat bands of muscular tissue or taeniae that separate rows of haustra or sacculations. Small protrusions, ‘warzen’ (warts), approximately 0.5 mm in diameter, can be seen on the mucosa in this section of colon. The second section of ascending colon is approximately 20 cm in length and has a single taenia and fewer, smaller haustra. The third portion of the ascending colon is termed the fusus coli and is a muscular area about 4 cm long. The fusus coli opens into the fourth section of ascending colon that is histologically indistinguishable from the transverse and descending colon. Because the fusus coli forms such a natural division between two morphologically and functionally distinct sections of the rabbit colon, the terms ‘proximal’ and ‘distal’ colon are sometimes used instead of ascending, transverse and descending colon (Snipes et al., 1982). The proximal colon includes the three taeniae section, the single taenia section and the fusus coli. The distal colon is 80–100 cm long and runs from the fusus coli to the rectum. (E) A ventral view of the ileocaecocolic complex, which occupies more than half of abdomen, mainly on the right side (see Figures 1.13–1.15). The complex has been slightly unrolled in order to illustrate its component parts. There are mesenteric attachments between the caecum, appendix, proximal colon, ileum, distal colon and descending duodenum. These organs form a complex three-dimensional structure in rabbits. The term ‘ileocaecocolic complex’ is used to describe the structure in this text. The body of the caecum has a spiral form consisting of one and a half turns, ending in an appendix that extends to the right flank. The axis of the spiral is the base of the caecum that receives the end of the ileum in the form of the sacculus rotundus. The ileum lies between the concavity of the body of the caecum and the convexity of the upper ascending colon and is attached to these two structures by peritoneal folds. Because of their peritoneal attachments to the spiral caecum, the ileum and upper ascending colon are also arranged in a spiral, and are integral components of the ileocaecocolic complex. The upper ascending colon begins as a smooth oval dilation, the ampulla coli, that forms the junction with the sacculus rotundus and the caecum. Parts of the descending colon and descending duodenum are attached to the distal end of the caecum by peritoneal folds. The left lobe of the pancreas lies in the peritoneal fold between the descending duodenum and descending colon.
Indigestible fibre passes down the colon to be rapidly eliminated as hard, dry faecal pellets. Smaller particles and fluids pass into the caecum where bacterial fermentation releases volatile fatty acids and synthesizes proteins and vitamins. Pellets of soft caecal contents (caecotrophs) are periodically expelled from the anus and re-ingested as a source of nutrients. This digestive strategy utilizes bacterial fermentation to synthesize nutrients and avoids the need to store large volumes of food in the digestive tract. Vegetation can be efficiently digested below ground without the need to spend long periods grazing and exposed to predators.
The rabbit’s characteristic of consuming caecotrophs directly from the anus is known as caecotrophy, although the term coprophagia is still used in some texts. Coprophagia is defined as ‘the ingestion of dung or faeces’ (Blood and Studdert, 1999). Faeces are defined as ‘body waste discharged from the intestine’ and so, strictly speaking, faecal material is not the substance that is ingested by rabbits as it is not waste material but nutritionally rich caecal contents. The terms soft faeces and night faeces are sometimes used to describe the capsules of caecal material known as caecotrophs. The term night faeces is misleading. Caecotrophs are produced during the day in wild rabbits. They are produced 4 to 8 h after feeding during a quiet undisturbed period, which is during the day for a wild rabbit in its burrow but can be during the night or early morning for a domestic or laboratory rabbit in its cage or hutch.
1.3.2 Ingestion of food
The rabbit has a wide visual field that allows it to watch for predators while it is grazing. The visual field does not include the area immediately under the nose. Food selection and ingestion is based on smell and from tactile information gained from the sensitive vibrissae around the nose and lips.
The teeth are adapted for the ingestion of a fibrous diet. All the teeth are open rooted and grow continuously. The incisors are adapted to cut through vegetation. The two large upper incisors have two tiny secondary incisors situated immediately behind them. The two lower incisors occlude just behind the upper primary incisors and wear against them to form a sharp cutting edge. There is a thick layer of enamel on the anterior aspect of the upper primary incisors but no enamel on the posterior aspect (Hirschfeld et al., 1973). The enamel on the lower incisors is evenly distributed on all aspects. The distribution of enamel in combination with the occlusal positioning of the upper and lower incisors allows the teeth to be constantly sharpened. Wild rabbits are capable of chewing through aluminium (Adams, 1987). The rate of growth of the upper incisors is approximately 2 mm per week (Shadle, 1936). Canine teeth are absent and there is a wide diastema between the incisors and the premolars and molars, which are grossly indistinguishable from each other. The premolars and molars form a row of five or six cheek teeth that are used for grinding the food before it is swallowed. The food is ground between the cheek teeth with jaw movements of up to 120 per minute (Brewer and Cruise, 1994).
Saliva is continuously secreted and contains amylase. Hunger is stimulated by a dry mouth and contractions of an empty stomach or by a decrease in blood levels of metabolites such as glucose, amino acid, lactic acid or volatile fatty acids (Fekete, 1989).
1.3.3 Anatomy and digestion in the stomach and small intestine
The stomach comprises about 15% of the volume of the gastrointestinal tract (Cruise and Brewer, 1994). It has a well-developed cardiac sphincter that prevents vomiting, and a muscular pyloric area, although in general the muscular layer of the stomach is weaker than in other species. There is always food material in the stomach. Together, the caecum and the stomach contain over 80% of the digesta (Lang, 1981a) and the amount of material in them is dependent on age, breed, diet and time of day. Water and large quantities of acid are secreted into the stomach. The postprandial pH can fall to 1–2, which effectively sterilizes ingesta before it passes into the small intestine. The stomach pH of suckling rabbits is higher at approximately 5–6.5, which permits the passage of bacteria through the stomach to the hindgut to colonize the caecum. During the digestion of caecotrophs the stomach pH rises to 3.0 (Blas and Gidenne, 1998). Transit time of food through the stomach is approximately 3–6 h (Carabaño and Piquer, 1998).
The duodenum begins with a slight enlargement that receives the bile duct. The right lobe of the pancreas is diffuse and is situated in the mesoduodenum of the duodenal loop. The body and the left lobe of the pancreas are much denser than the right lobe. The left lobe lies between the stomach and the transverse colon and extends as far as the spleen. A single pancreatic duct opens at the junction of the transverse and ascending loops of the duodenum (see Figure 1.3B). This is the accessory pancreatic duct. The terminal part of the main pancreatic duct disappears during embryonic development. The accessory pancreatic duct communicates with both pancreatic lobes. The jejunum is long and convoluted. The end of the ileum is expanded into a spherical thick-walled enlargement known as the sacculus rotundus that forms the junction between the ileum, caecum and proximal colon. The sacculus rotundus is unique to the rabbit and has abundant aggregations of lymphoid tissue and macrophages in the lamina propria and submucosa. An ileocolic valve controls movement of digesta from the ileum into the sacculus rotundus and also prevents reverse flow into the small intestine. Motilin, a polypeptide hormone secreted by enterochromaffin cells of the duodenum and jejunum, stimulates gastrointestinal smooth muscle. Fat stimulates and carbohydrate inhibits its release. In the small intestine, motilin activity is decreased aborally. It disappears in the caecum and reappears in the colon and rectum (Brewer and Cruise, 1994).
Digestion and absorption of nutrients in the stomach and small intestine are similar to other monogastric animals. Caecotrophs are digested in this section of the gastrointestinal tract. Caecotrophs contain micro-organisms and are the products of microbial fermentation such as amino acids, volatile fatty acids and vitamins. They are encapsulated in a gelatinous mucous coating that protects them from the acidity of the stomach. Some fermentation takes place within the caecotrophs as they lie in the gastric fundus for 6–8 hours before being digested. Lysozyme is secreted by the colon and incorporated into the caecotroph during its passage through the large intestine (Camara and Prieur, 1984). The bacteriolytic activity of lysozyme enables microbial protein to be degraded and absorbed from the small intestine in addition to the amino acids and vitamins present in the caecotrophs. Amylase is produced by bacteria within the caecotroph that converts glucose into carbon dioxide and lactic acid, which is absorbed from the stomach and small intestine (Fekete, 1989).
Hydrochloric acid and pepsin initiate digestion in the stomach that continues in the small intestine in a manner similar to that of other mammals. Pancreatic amylase production is relatively modest. There are alternative sources of amylase such as saliva and caecotrophs. In rabbits, ligation of the pancreatic duct does not result in pancreatic insufficiency (Brewer and Cruise, 1994). Proteolytic enzymes and chymotrypsin can be found in the intestinal lumen within a few weeks of the operation. It is thought that small pancreatic ducts that connect directly with the duodenum are the source of the enzymes. Bicarbonate is secreted into the duodenum and neutralizes the acidic digesta as it leaves the stomach. In the jejunum bicarbonate is absorbed rather than secreted. Transit time through the small intestine is fast. Estimated retention times in the jejunum and ileum are 10–20 and 30–60 minutes, respectively (Carabaño and Piquer, 1998).
1.3.4 Anatomy of the hindgut
The anatomy of the rabbit’s digestive system is illustrated in detail by Barone et al. (1973) and Barone (1997). A schematic representation of the rabbit’s digestive system is given in Figure 1.3. The ileocaecocolic segment is illustrated in Figure 1.3E and the topographical anatomy of the small intestine and colon is described in Figure 8.2.
The sacculus rotundus opens into the ampulla caecalis coli, which forms a T-junction between the ileum, caecum and proximal colon. The ampulla caecalis coli, caecum and proximal colon are specially adapted for mixing and separating large quantities of food. Large particles of indigestible fibre are separated from small fermentable particles and fluid. The large particles are sent distally along the colon while the small particles and fluid are sent proximally into the caecum where bacterial fermentation takes place (Figure 1.4). The thin-walled caecum ends in a narrow blind appendix that is heavily endowed with lymphoid tissue. The appendix is often described as ‘vermiform’ due to its worm-shaped appearance. The gut-associated lymphoid tissue (GALT) of the rabbit is predominantly in the hindgut and represents over 50% of the total lymphoid tissue, which may account for the relatively small spleen of rabbits (Percy and Barthold, 1993).
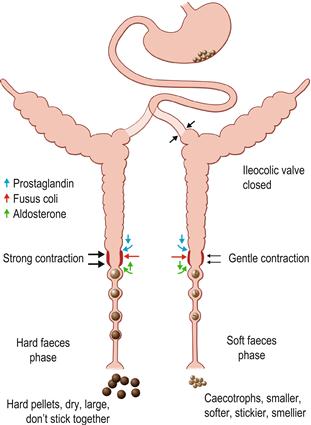
Figure 1.4 The activity of the digestive system during excretion of hard and soft faeces.
(A) The motility and function of the hindgut can change depending on the type of faeces formed within the colon. The formation of hard faeces is known as the hard faeces phase and the expulsion of caecotrophs is known as the soft faeces phase. The phases of excretion follow a marked circadian rhythm. The hard faeces phase is shown in black. The soft faeces phase is shown in green. Exchange of water, electrolytes and nutrients across the intestinal epithelium alter with the phase of faeces excretion. The direction of water and electrolyte exchange is indicated by arrows. The proximal part of the ascending colon is able to separate digesta into two fractions that are simultaneously sent in opposite directions. During the hard faeces phase, water is secreted into the proximal colon and the intestinal contents are thoroughly mixed by contractions of the caecum and colon. Large indigestible particles (> 0.5 mm) tend to accumulate in the lumen of the proximal part of the ascending colon and are moved distally, whereas smaller particles accumulate at the circumference in the sac-like haustra. Haustrum is the Latin term for a pump. Haustral activity sends the small particles and fluid proximally into the caecum where bacterial fermentation takes place. The indigestible fraction, composed of large particles, is moved rapidly through the proximal colon to the fusus coli and distal colon where it is formed into hard, round, dry pellets that are excreted from the anus. Rhythmic caecal contractility is greatest during the hard faeces phase. (B) Periodically, the motility of the caecum and proximal colon alters completely. Haustral activity ceases and the caecum contracts, sending caecal material swiftly along the large intestine. In the fusus coli the material is formed into soft pellets that become encapsulated in mucus (see Figure 1.5). This is the soft faeces phase of excretion when caecotrophs pass through the colon to be expelled from the anus. Expulsion of caecotrophs coincides with a decrease in rhythmic motility of the caecum and proximal colon, and increase in motility of the distal colon. Soft faeces or caecotrophs are expelled once or twice daily, at least 4 h after feeding, usually during periods of rest. The transit time for soft faeces through the colon is 1.5–2.5 times faster than that for hard faeces. Motility in the upper gastrointestinal tract remains the same during the hard and soft faeces phases. The differences in colonic motility during the hard and soft faeces phase of excretion are most pronounced in the second section of proximal colon that has a single row of haustra. The fusus coli is a specially adapted area of the colon that acts as a differential pacemaker for the initiation of peristaltic waves in the proximal and distal colon that alter with the phase of faeces excretion. The fusus coli is highly innervated and is influenced by hormones such as aldosterone and prostaglandins. During the hard faeces phase, the intestinal contents lose considerable quantities of water, potassium and sodium during their passage through the fusus coli. Water is mechanically squeezed out of the fibrous material before it passes to the distal colon where absorption of water, volatile fatty acids and electrolytes continues, leaving the residue of dry, indigestible matter that is expelled as hard faecal pellets.
The ascending colon of the rabbit is divided into four sections. At the proximal end, the ampulla caecalis coli opens into the first section, which is approximately 10 cm long and has three longitudinal flat bands of muscular tissue or taeniae separating rows of haustra or sacculations. Small protrusions, approximately 0.5 mm in diameter, can be seen on the mucosa in this section of colon. These cauliflower-like protrusions have been termed warzen (warts) and are believed to be unique to lagomorphs. They represent an increase in the surface area of the colon that would favour increased absorption. The protrusions may also assist mechanical separation of intestinal contents. Histologically, the muscular layers of the taenia contain many autonomic fibres that are part of the myenteric plexus (Snipes et al., 1982). The second section of ascending colon is approximately 20 cm in length and has a single taenia and fewer, smaller haustra. There is an abundance of myenteric plexus in this region. The third portion of the ascending colon is termed the fusus coli and is a muscular area about 4 cm long (see Figure 1.5). This area is highly innervated and vascular. The mucosal surface of the fusus coli is distinguished by prominent longitudinal folds and contains numerous goblet cells. The fusus coli opens into the fourth section of ascending colon, which is histologically indistinguishable from the transverse and descending colon. Because the fusus coli forms such a natural division between two morphologically and functionally distinct sections of the rabbit colon, many physiological texts have abandoned the traditional description of ascending, transverse and descending colon and use the terms proximal and distal colon instead (Snipes et al., 1982). The proximal colon includes the three taeniae section, the single taenia section and the fusus coli. The distal colon is 80–100 cm long and runs from the fusus coli to the rectum. The mucosa of the distal colon is smooth with no surface specialization. The tunica mucosa possesses short crypts with numerous goblet cells reaching into the base. This section of the colon is thin-walled and usually contains hard faecal pellets.
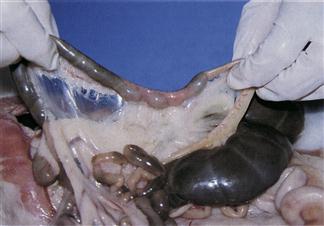
Figure 1.5 Fusus coli.
The fusus coli is a highly innervated, vascular, muscular section of the ascending colon (see Section 1.3.5). The fusus coli acts as a pacemaker for colonic motility that alters with the type of faeces that are passing through the colon. It is influenced by the autonomic nervous system and hormones such as aldosterone and prostaglandins. The mucosa of the fusus coli is deeply folded and contains many goblet cells. This plate shows the fusus coli of a rabbit that died during the morning when the colon was in the soft faeces phase. Pasty caecal material is passing into the fusus coli from the proximal colon (left). In the fusus coli, the intestinal contents are squeezed into pellets that become encapsulated in mucus before being excreted as soft caecotrophs.
1.3.5 Motility of the hindgut
The motility and function of the hindgut can change, depending on the type of faeces formed within the colon. The formation of hard faeces is known as the hard faeces phase and coincides with feeding activity. The expulsion of caecotrophs is known as the soft faeces phase. The phases of excretion follow a marked circadian rhythm. In caged rabbits with ad lib access to food, feed intake increases from 15.00 to 18.00 h and remains high until midnight. Intake then reduces until 02.00, when a new phase starts, with a maximum at 06.00, ending at 08.00 when the soft faeces phase begins. This natural pattern of feeding behaviour and faecal excretion can be seen in pet rabbits, although it may be altered by type and availability of food, age, pregnancy and lactation (Carabaño and Piquer, 1998).
During the hard faeces phase, water is secreted into the proximal colon, which aids the process of mixture and separation. Intestinal contents are thoroughly mixed by contractions of the caecum and colon that separate the digesta into large indigestible particles, and small particles including bacteria and water-soluble components. The indigestible fraction is moved rapidly through the proximal colon to the fusus coli and distal colon before being excreted from the anus. The fermentable fraction is moved in a retrograde direction back into the caecum. The large indigestible particles (> 0.5 mm) tend to accumulate in the lumen of the proximal part of the ascending colon and are moved distally, whereas smaller fermentable particles accumulate at the circumference in the sac-like haustra. Haustral activity sends the small particles proximally into the caecum. Caecal contractility is greatest during the hard faeces phase when the liquid intestinal contents are mixed and separated in the proximal colon. Periodically, the motility of the caecum and proximal colon alters completely. Haustral activity ceases and caecal material is moved swiftly along the large colon. In the fusus coli the material is then separated into pellets that become encapsulated in mucus. This is the soft faeces phase of excretion. Soft faeces or caecotrophs are expelled at least 4 h after feeding, usually during periods of rest.
The fusus coli is a specially adapted area of the colon that acts as a differential pacemaker for the initiation of peristaltic waves in the proximal and distal colon (Ruckesbusch and Fioramonti, 1976). The nature and direction of the peristaltic waves alter with the phase of faeces excretion. The fusus coli is highly innervated and is influenced by hormones such as aldosterone and prostaglandins. During hard faeces production aldosterone levels are high, but they fall during the soft faeces phase of excretion. Prostaglandins inhibit motility of the proximal colon and stimulate the distal colon, aiding the elimination of soft faeces or caecotrophs (Pairet et al., 1986).
Three types of contractions occur in the proximal colon. Haustral activity results from high-frequency repetitive contractions of the haustral walls that last about 3 seconds and coincide with orally migrating shallow annular constrictions. Segmental activity is the result of low-frequency deep annular constrictions that move aborally and last about 14 seconds. The third type of contraction of the proximal colon is a monophasic progressive wave of peristaltic contractions. These peristaltic contractions last about 5 seconds during the hard faeces phase and 1.5 seconds during the soft faeces phase (Ehrlein et al., 1982). Expulsion of caecotrophs coincides with a decrease in motility of the caecum and proximal colon and an increase in motility of the distal colon. The transit time for caecotrophs through the colon is 1.5–2.5 times faster than that for hard faeces (Fioramonti and Ruckesbusch, 1976). Motility in the upper gastrointestinal tract remains the same during the hard and soft faeces phases, with slow contractions of the small intestine occurring every 10–15 minutes (Ruckesbusch et al., 1985). The differences in colonic motility during the hard and soft faeces phase of excretion are most pronounced in the second section of proximal colon that has a single layer of haustra.
During the hard faeces phase, the intestinal contents lose considerable quantities of water, potassium and sodium during their passage through the fusus coli (Snipes et al., 1982). The compression of intestinal contents into faecal pellets during the hard faeces phase can be correlated with the strong muscular wall of the fusus coli and its dense innervation. Water is mechanically squeezed out of the fibrous material before it passes to the distal colon where absorption of water, volatile fatty acids and electrolytes continues, leaving a residue of dry, indigestible matter that is expelled as hard, dry faecal pellets.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




