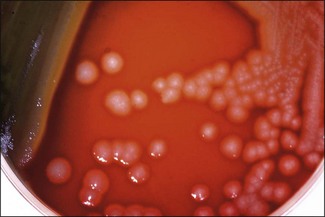
Chapter 18 The genus Pseudomonas was originally organized into five major species clusters (rRNA homology groups). However, this classification has undergone revision and Pseudomonas species have now been reclassified into many different genera. In addition to Pseudomonas, two of these genera are of significance in veterinary medicine: Burkholderia and Stenotrophomonas. The genus Pseudomonas (sensu stricto) represents the rRNA homology group 1 with the type species Pseudomonas aeruginosa which is the most important veterinary pathogen in the genus Pseudomonas followed by P. fluorescens. Two species of the genus Burkholderia (formerly rRNA group II pseudomonads), B. mallei and B. pseudomallei, are generally recognized as important animal or human pathogens. The genus Stenotrophomonas has one species of clinical veterinary significance, S. maltophilia (formerly Pseudomonas maltophilia or Xanthomonas maltophilia) (Versalovic 2011). Pseudomonas and Burkholderia species are medium-sized (0.5–1 µm × 1.5–5 µm) straight or slightly curved Gram-negative rods. Stenotrophomonas species tend to be straight and slightly smaller (0.4–0.7 µm × 0.7–1.8 µm). These bacteria are strict aerobes, non-spore-forming, oxidative, catalase-positive, oxidase-positive (except P. oryzihabitans, P. luteola and the genus Stenotrophomonas) and most are motile by one or several polar flagella (except B. mallei). Some species produce soluble pigments and most will grow on MacConkey agar as lactose non-fermenters as well as converting nitrate to nitrite or nitrogen gas. Pseudomonas, Stenotrophomonas and Burkholderia species have a worldwide distribution. These bacteria are environmental microorganisms typically found in water, soil, on plants, fruits and vegetables. They are opportunistic pathogens of animals, humans and plants. They have a predilection for aqueous environments, surviving well in them. As a result, they can be problematic in hospital settings. Potential sources of P. aeruginosa are diverse including disinfectants, ointments, soaps, eye drops, irrigation fluids and equipment. This bacterium is frequently found in aerators and traps of sinks. As P. aeruginosa is resistant to many antimicrobials, it frequently causes infection in animals undergoing antibiotic treatment or in immunocompromised hosts. Pseudomonas aeruginosa is found infrequently as part of the microbial flora of healthy animals. In these animals, it can be found on skin and mucous membranes, particularly the gastrointestinal tract. Pseudomonas fluorescens, present in soil and water, is associated with food spoilage and lesions in reptiles and fish (Sakai et al. 1989; Swain et al. 2007). Burkholderia mallei, the aetiologic agent of glanders, is a listed disease by the Office International des Épizooties (OIE), also known as the World Organization for Animal Health. Glanders is now rare as there has been considerable success in the global eradication of this disease, principally owing to the fact that B. mallei is an obligate parasite with a restricted host range and, in addition, effective tests are available to detect carriers of the infection. Infected Equidae are the reservoir for B. mallei. This organism is unable to survive in the environment for more than two weeks. It is easily killed by desiccation, sunlight and common disinfectants. Burkholderia mallei once had a wide geographical distribution but now is mainly seen in China and Mongolia with pockets of infection in India, Iraq, Turkey and the Philippines. Burkholderia pseudomallei, the cause of melioidosis, is found primarily in tropical and subtropical regions; particularly in the rice-growing areas of Thailand, Vietnam and India; but also in the Northern Territory of Australia (Edmond et al. 2001). The disease usually occurs in tropical regions between 20° northern and southern latitudes but melioidosis has been reported in localized areas of France, Iran, China and the USA. Stenotrophomonas maltophilia is readily isolated from water, soil and sewage. It has emerged as an opportunistic pathogen in animals and immunocompromised humans. Stenotrophomonas maltophilia is resistant to many antimicrobials (Denton & Kerr 1998) and mainly causes hospital-acquired infections in humans. The respiratory tract is the most common site of infection. This microorganism has also been isolated from the semen of boars and bulls, diminishing semen quality and viability and resulting in impaired fertilization and embryonic development in vitro (Bielanski et al. 2003, Althouse & Lu 2005). Stenotrophomonas maltophilia is part of the normal microflora of the mouth and cloacae of healthy snakes (Hejnar et al. 2007). Burkholderia mallei is the causative agent of glanders, a disease of livestock that particularly affects horses, mules, and donkeys (Table 18.1). This bacterium is a highly pathogenic microorganism for both humans and animals. Burkholderia pseudomallei is the aetiological agent of melioidosis, a disease in which treatment failures and relapses are common, with pneumonia as the most common clinical presentation. Both of these species have been identified as potential agents of bioterrorism (category B biothreat agents). Pseudomonas aeruginosa and S. maltophilia are both considered opportunistic pathogens and can cause a variety of infections (Table 18.1). A number of saprophytic Pseudomonas species and Burkholderia species have been implicated in occasional infections of animals (Jackson & Phillips 1996, Berriatua et al. 2001, Matchett et al. 2003) including P. fluorescens (septicaemia in rainbow trout and Tilapia and necrotizing hepatitis in pet birds), P. putida (endotoxic shock in a cynomolgous macaque) and B. cepacia, formerly P. cepacia (outbreak of subclinical mastitis in a flock of dairy sheep). Table 18.1 Main diseases caused by the major pathogenic Pseudomonas, Burkholderia and Stenotrophomonas species in veterinary medicine Pseudomonas aeruginosa is rarely involved in primary disease. Predisposing causes include trauma to tissue (burns and wounds), debilitation due to malignancy or immunodeficiency and an imbalance in the normal flora, often caused by antibiotic therapy. Pseudomonas aeruginosa possesses cell-associated virulence factors such as pili, flagella, lipopolysaccharide and alginate/biofilm. It also produces a number of extracellular products such as protein exotoxin A, proteases, type III secretion system exoenzymes, rhamnolipid, phospholipase C, and siderophores (pyochelin, pyocyanin, and pyoverdin). These virulence factors all play a role in disease pathogenesis (Table 18.2). Flagella provide motility and act as adhesins to mucin and respiratory epithelial cells. Pili are the major adhesins implicated in the initial attachment phase to host tissues. P. aeruginosa occurs in both rough and smooth lipopolysaccharide (LPS) forms (Sadovskaya et al. 2000). LPS is involved in adherence and invasion and its lipid A part mediates inflammation and tissue damage. Under particular conditions, P. aeruginosa can produce an alginate structure which is a slime-like, mucoid exopolysaccharide. This structure can form a viscous gel surrounding the bacteria and help in the generation of biofilms involved in adherence. It also protects the bacterium from phagocytosis. The type III secretion system consists of bacterial proteins which act as a syringe to deliver cytotoxins into the cytoplasm of host cells. The toxins are involved in epithelial cell damage and in the inhibition of phagocytic cells. Siderophores are involved in iron acquisition and promote survival in low-iron conditions such as host tissues. Interestingly, pyocyanin can colour pus and stain wool a greenish blue. Table 18.2 Main virulence factors of Pseudomonas aeruginosa Virulence factors such as capsular material, LuxI and LuxR quorum-sensing signals, a possible antigenic variation system and a type III secretion system have been reported for B. mallei. Its capsular polysaccharide is reported as a major virulence factor (DeShazer et al. 2001) and one of the factors facilitating its persistence in the body. The bacterium is capable of intracellular survival. Many Gram-negative pathogens regulate virulence factor expression by using a cell density mechanism termed quorum sensing. Burkholderia mallei produces several acyl-homoserine lactones (acyl-HSLs) which serve as quorum-sensing signals (Ulrich et al. 2004). Genomic analysis of B. mallei has identified a number of putative virulence factors. The genome contains numerous insertion sequence elements and a vast number of simple sequence repeats. It is likely that variation in simple sequence repeats in key genes provide a mechanism for generating antigenic variation. This may account for the mammalian host’s inability to build a durable adaptive immune response to B. mallei (Nierman et al. 2004). Mutagenesis experiments have shown that a functional type III secretion system is required for the full pathogenicity of B. mallei in animal models of infection (Ulrich & DeShazer 2004). A major function of this secretion system is to secrete virulence-associated proteins into target cells of the host organism. Burkholderia pseudomallei is motile via its flagella. It also produces several other potential virulent factors such as extracellular proteases, serine metalloprotease, haemolysin, lipase, lecithinase, endotoxin, lethal toxins, and surface capsule-like structures. The toxins include a lethal factor with anticoagulant activity and a skin-necrotizing proteolytic agent. Malleobactin is a siderophore involved in iron acquisition (Alice et al. 2006). Studies have indicated that virulence of selected B. pseudomallei isolates is variable and dependent on factors such as iron bioavailability, inoculum size and host risk factors (Ulett et al. 2001). Stenotrophomonas maltophilia is also considered an opportunistic pathogen which can cause a variety of infections in veterinary medicine. In humans, it is considered as an emerging nosocomial bacterial pathogen which is being isolated with increasing frequency from the airways of cystic fibrosis patients. Stenotrophomonas maltophilia-associated infection is problematic because many strains of the bacterium are resistant to multiple antibiotics. Other virulence factors of note include the ability to form a biofilm, adherence and the ability to invade respiratory epithelial cells (Di Bonaventura et al. 2007). Direct microscopy from specimens is of little diagnostic use as Pseudomonas, Burkholderia and Stenotrophomonas are medium-sized, Gram-negative rods with no other distinctive characteristics. However, direct microscopy of Gram-stained smears with B. pseudomallei will often reveal small Gram-negative bacilli with bipolar staining, ‘safety pin’ appearance. A fluorescent antibody technique may be useful for B. mallei and B. pseudomallei (Walsh et al. 1994). The colonies of P. aeruginosa are large (3–4 mm), flat, spreading, greenish-blue with a serrated edge and a characteristic fruity, grape-like odour of aminoacetophenone. Colonial variation includes smooth, soft and shiny (S-forms), dwarf, dry and granular (R-forms) not unlike some colonies of Bacillus species, and mucoid (M-forms) that are frequently biochemically atypical. Most strains give a clear zone of haemolysis on blood agar (Fig. 18.1). Pyocyanin, a bluish pigment unique to P. aeruginosa, gives the blue-green colour associated with many cultures. Some strains have colonies with a distinctive metallic sheen (Fig. 18.2). Pseudomonas aeruginosa produces large, pale colonies on MacConkey agar (unable to utilize lactose) with greenish-blue pigment superimposed (Fig. 18.3). Red colonies and medium, indicative of an alkaline reaction, are seen on brilliant green (Fig. 18.2) and XLD agars. No H2S is produced on XLD medium. Strains of P. aeruginosa produce the water-soluble diffusible pigments pyocyanin (blue, phenazine pigment), pyoverdin (water-soluble yellow-green or yellow-brown pigment), pyorubin (red) and pyomelanin (dark brown) (Fig. 18.4) in varying combinations and amounts. When pyoverdin is combined with pyocyanin, the bright green colour characteristic of P. aeruginosa is expressed. Some strains produce all four pigments. Pyorubin and pyomelanin are less commonly produced, develop slowly and are seen best by growing the strains on nutrient agar slants at room temperature for up to two weeks. As pyocyanin is unique to P. aeruginosa this is an important diagnostic characteristic although strains vary in the amount of the pigment they produce. Media such as Pseudomonas agar P (BD Diagnostics) (Fig. 18.5) will enhance pyocyanin production and Pseudomonas agar F (BD Diagnostics) enhances pyoverdin production (Fig. 18.6). Pyoverdin, once called ‘fluorescin’, will fluoresce under ultra-violet light. Pseudomonas aeruginosa is classified as a member of the fluorescent pseudomonad group which produce pyoverdin. Some strains of P. aeruginosa do not produce pigments and are highly mucoid. These may also be atypical in certain biochemical reactions, making them difficult to identify. Figure 18.1 Pseudomonas aeruginosa on sheep blood agar showing large, flat, irregular-edged colonies resembling those of some Bacillus species. The green-blue pyocyanin pigment is most obvious in areas of heaviest growth.
Pseudomonas, Burkholderia and Stenotrophomonas species
Genus Characteristics
Natural Habitat
Pathogenesis and Pathogenicity
Virulence determinants
Functions
Exotoxin A (ADP-ribosyl-transferase)
Cytotoxic, invasion of tissue and cellular damage, immunosuppressive action
Flagellum
Motility, adherence to mucin
Elastase (LasB and LasA)
Damage to tissues of the lungs and blood vessels
Alkaline protease
Tissue damage
Phospholipase C (haemolysin)
Tissue damage, stimulation of inflammatory mediators
Siderophores (pyoverdin, pyocyanin, pyochelin)
Iron uptake
Rhamnolipid (haemolysin with lecithinase activity)
Damage to host cell membranes and impaired mucociliary clearance
Type III secretion system (exoenzymes S, T, U and Y)
Damage to host tissues, cytotoxic, implicated in invasion process
Alginate-biofilm
Protection from phagocytosis, adhesin, antimicrobial resistance
LPS
Adherence to epithelial cells and invasion, resistance to phagocytosis, serum resistance, and production of proinflammatory cytokines
Pili
Adherence to epithelial cells and mucin
Laboratory Diagnosis
Direct microscopy
Identification
Colonial morphology
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pseudomonas, Burkholderia and Stenotrophomonas species
Only gold members can continue reading. Log In or Register to continue
