Chapter 4. Protein and Amino Acids
Proteins are complex molecules that, like carbohydrates and fats, contain carbon, hydrogen, and oxygen. In addition, all proteins contain the element nitrogen, and the majority contain sulfur. All proteins contain approximately 16% nitrogen. This consistency led to the development of the nitrogen balance test, which has been used traditionally to estimate an animal’s body protein status. Nitrogen balance tests measure intake and excretion of nitrogen in animals that are fed a test diet. Net loss or gain of nitrogen then indicates increases or decreases in total body protein reserves (see Section 2, p. 89). Amino acids are the basic units of proteins and are held together by peptide linkages to form long protein chains (Figure 4-1 and Figure 4-2). Proteins can range in size from several amino acids to large, complex molecules that consist of several intricately folded peptide chains, and can be classified as either simple or complex forms. Once hydrolysis begins, simple proteins yield only amino acids or their derivatives. Examples include albumin in blood plasma, lactalbumin in milk, zein in corn, and the structural proteins keratin, collagen, and elastin. Complex or conjugated proteins are made up of a simple protein combined with a nonprotein molecule. Examples of complex proteins include the nucleoproteins, glycoproteins, and phosphoproteins.
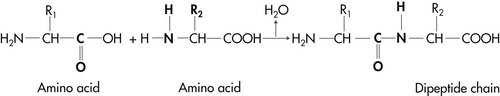 |
| Figure 4-1 |
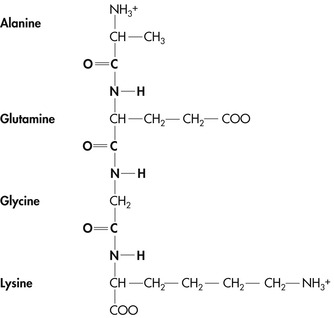 |
| Figure 4-2 |
Proteins in the body have numerous functions. They are the major structural components of hair, feathers, skin, nails, tendons, ligaments, and cartilage. The fibrous protein collagen is the basic material that forms most of the connective tissue throughout the body. Contractile proteins such as myosin and actin are involved in regulating muscle action. All of the enzymes that catalyze the body’s essential metabolic reactions and are essential for nutrient digestion and assimilation are also protein molecules. Many hormones that control the homeostatic mechanisms of various systems in the body are composed of protein; for example, insulin and glucagon are two protein hormones involved in the control of normal blood glucose levels. Proteins found in the blood act as important carrier substances. These substances include hemoglobin, which carries oxygen to tissues; transferrin, which transports iron; and retinol-binding protein, which carries vitamin A. In addition to their transport functions, plasma proteins also contribute to the regulation of acid-base balance. Finally, the body’s immune system relies on protein substances; the antibodies that maintain the body’s resistance to disease are all composed of large protein molecules.
Proteins are the major structural components of the body. Enzymes essential for nutrient digestion are proteins, as are many hormones, such as insulin and glucagon. Blood proteins act as important carrier substances and contribute to the regulation of acid-base balance. Finally, the body’s immune system relies on protein substances; the antibodies that maintain the body’s resistance to disease are all composed of large protein molecules.
Protein present in the body is not static; it is in a constant state of flux involving degradation and synthesis. Although tissues vary greatly in their rate of turnover, all protein molecules in the body are eventually catabolized and replaced. During growth and reproduction, additional protein is needed for the accretion of new tissue. A regular influx of protein and nitrogen, supplied by the diet, is necessary to maintain normal metabolic processes and provide for tissue maintenance and growth. The body has the ability to synthesize new proteins from amino acids, provided that all of the necessary amino acids are available to the tissue cells. At the tissue and cellular level it is inconsequential whether the amino acids that are present were synthesized by the body, supplied from the diet as single amino acid units, or supplied from the diet in the form of intact protein. Therefore it is correct to state that the body does not really have a protein requirement per se but rather has a requirement for certain amino acids and a level of nitrogen. This requirement is still addressed as a protein requirement in the diet because most practical diets contain intact protein sources, not individual amino acids.
There are 22 alpha-amino acids found in protein chains. The term alpha denotes the attachment of the amino group (NH 2) to the first (alpha-) carbon in the molecule. Of these 22 alpha-amino acids, if an adequate source of nitrogen is supplied in the diet, dogs and cats are able to synthesize 12 at a sufficient rate to meet the body’s needs for growth, performance, and maintenance. These are called the nonessential, or dispensable, amino acids, and they may be either supplied in the diet or synthesized by the body. The remaining 10 amino acids cannot be synthesized at a rate that is sufficient to meet the body’s needs. These are the essential amino acids, and they must be supplied in the pet’s diet. In addition to these 10, the cat has an additional requirement for taurine, a beta-sulfonic acid (see Section 2, pp. 97-99). The essential and nonessential amino acids are listed in Box 4-1.
BOX 4-1
| E ssential amino acids | N onessential amino acids |
|---|---|
| Arginine | Alanine |
| Histidine | Asparagine |
| Isoleucine | Aspartate |
| Leucine | Cysteine |
| Lysine | Glutamate |
| Methionine | Glutamine |
| Phenylalanine | Glycine |
| Taurine (cats only) | Hydroxylysine |
| Threonine | Hydroxyproline |
| Tryptophan | Proline |
| Valine | Serine |
| Tyrosine |
Dietary protein serves several important functions. It provides the essential amino acids, which are used for protein synthesis in the growth and repair of tissue, and it is the body’s principal source of nitrogen. Nitrogen is essential for the synthesis of the nonessential amino acids and of other nitrogen-containing molecules, such as nucleic acids, purines, pyrimidines, and certain neurotransmitter substances. Amino acids supplied by dietary protein can also be metabolized for energy. The gross energy (GE) of amino acids is 5.65 kilocalories per gram (kcal/g). When fecal and urinary losses are accounted for, the metabolizable energy (ME) of protein in dog and cat diets is approximately 3.5 kcal/g; that is, approximately the same amount of energy supplied by dietary carbohydrate. Animals are unable to store excess amino acids. Surplus amino acids are either used directly for energy or are converted to glycogen or fat for energy storage. An ancillary function of the protein in dog and cat diets is to provide a source of flavor. Different flavors are created when food proteins are cooked in the presence of carbohydrate and fat. 1 In general, as the protein content of a dog or cat diet increases, so does its palatability and acceptability.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


