Chapter 28. Development and Treatment of Obesity
Obesity is the most common form of malnutrition seen in companion animals in the United States and other industrialized countries. Although incidence estimates vary depending upon the population that was surveyed and methodology used, there is consensus that between 20% and 50% of dogs living in homes are overweight or obese. 1.2. and 3. Using the lowest value of 20%, the translation of this figure into actual dog numbers is sobering. Of the 72 million dogs living in homes in the United States today, at least 14 million are obese. 4 Similarly, recent studies of house cats report that between 25% and 50% of the cats seen by veterinarians are overweight or obese. 5.6. and 7. This means that at least 21 million of the 82 million cats living in U.S. homes are overweight. Middle-aged adults (5 to 8 years) are most likely to be above their ideal body weight, while fewer young adults and geriatric pets are overweight. 8 Similar to humans, dogs that are overweight when they are adolescents are more likely to gain excessive amounts of weight as adults. 9 It is theorized that the incidence of obesity in companion animals has increased because a sedentary lifestyle has become the norm rather than the exception for many. In addition, the provision of highly palatable and energy-dense foods may further contribute to the energy imbalance that leads to obesity.
Unfortunately, like contemporary American humans, a large proportion of cats and dogs, between 25% and 50%, are overweight or obese. Living a sedentary lifestyle, along with overfeeding highly palatable and energy-dense foods, may be important contributing factors.
DEFINITION AND HEALTH RISKS
Obesity is defined as the excessive accumulation of fat in the adipose storage areas of the body, eventually contributing to adverse effects on health and mortality. 10 Dogs and cats that are 10% to 20% above their ideal body weight are generally classified as overweight, and those whose body weight is greater than 20% above normal are classified as obese. 6 This standard is corroborated by evidence in human subjects that health problems associated with overweight conditions begin to increase when weight is 15% or greater above ideal. Similar to humans, numerous health risks are associated with overweight conditions in dogs and cats.
Several metabolic aberrations are associated with obesity in dogs and cats. Obese dogs and cats frequently develop glucose intolerance and abnormal basal insulin and insulin response curves. 11. and 12. Persistent hyperinsulinemia is theorized to be an important factor in the eventual development of diabetes mellitus in overweight pets; overweight cats are more than three times more likely to develop diabetes mellitus than normal weight cats. 13 Recent research shows that these changes are influenced by the increased production of adipocyte cytokines by fat cells and associated chronic inflammatory responses. 14 When body weight is reduced in obese dogs, glucose intolerance often improves to near-normal values. 15 Obesity in dogs is also associated with alterations in blood lipids, including increased plasma cholesterol and triglyceride concentrations. 16. and 17. Although these values do not necessarily exceed the upper limit of the reference range for dogs, they may be associated with other obesity-related health problems.
Obesity may contribute to the development of pulmonary and cardiovascular disease in dogs. 18 Excess weight puts a strain on the circulatory system because increased cardiac workload is required to perfuse the increased tissue mass. This increased workload may cause additional stress to a heart that is already weakened by fatty infiltration. Changes in pulmonary health lead to difficulties breathing (airway dysfunction) both during and after exercise. 19 There is also some evidence suggesting that overweight conditions contribute to secondary hypertension in dogs, although there is controversy about this relationship. 20. and 21. Studies in cats have not been conducted, but it is theorized that a similar effect may be found in this species.
Physical effects of carrying excess weight contribute to exercise and heat intolerance, joint and locomotor problems, and the development of osteoarthritis and chronic lameness. In a long-term prospective study, both the frequency and severity of osteoarthritis were greater in Labrador Retrievers that were fed ad libitum during the first several years of life than in dogs that were fed 25% less food and that weighed significantly less. 22 Increased weight was significantly and positively correlated with increased incidence and severity of osteoarthritis. Osteoarthritic changes also developed earlier in life in dogs that were overweight than in lean dogs. Similarly, cats that are overweight are more likely to show reduced mobility and to develop lameness than cats that are in optimal condition. 13
In cats, there is an association between overweight conditions and certain types of dermatoses, such as feline acne, alopecia, and scale formation. These changes are presumed to be related to a reduced ability to self-groom and in some cases to the development of pressure sores from reduced activity. Other potential health risks to cats include hepatic lipidosis (specifically in the event of rapid weight loss) and feline lower urinary tract disease (FLUTD). 6. and 23.
A link between obesity and certain types of cancer has been reported in both dogs and cats by some authors. For example, one study reported that female dogs that were obese prior to a diagnosis of mammary carcinoma experienced increased risk of death from the disease when compared with dogs that were not overweight at the time of diagnosis. 24 Another study reported that being obese early in life increased a dog’s risk of developing mammary tumors. 25 Conversely other researchers failed to find an association between canine mammary carcinoma and body weight. 26 Additional prospective studies that include larger groups of animals are needed to better understand this relationship and to tease out possible differences between types of cancer and their relationship to body condition.
Finally, obese dogs and cats are difficult to thoroughly evaluate during veterinary examinations because of the presence of overlying layers of adipose tissue and difficulty obtaining blood. Diagnostic imaging such as ultrasonography is also impeded in overweight animals. Like humans, overweight animals have an increased surgical and anesthetic risk, and experience increased incidence of morbidity and mortality following surgical procedures. For example, surgical time to complete ovariohysterectomy is significantly longer in overweight females than in lean dogs. 27 It is without question that altogether the numerous health risks associated with overweight conditions in companion animals negatively affect a pet’s quality of life and contribute to both morbidity and mortality (Box 28-1). 28
BOX 28-1
Glucose intolerance and abnormal insulin response
Alterations in blood lipids (elevated cholesterol and triglyceride concentrations)
Increased risk for pulmonary and cardiovascular disease
Reduced exercise and heat tolerance
Joint and locomotor (mobility) problems
Reduced ability to self-groom and increased risk for dermatoses in cats
Increased surgical and anesthetic risks for morbidity and mortality
Possible increased risk for certain forms of cancer
Reduced quality of life
Health risks associated with overweight conditions in dogs and cats include hyperinsulinemia, glucose intolerance, diabetes mellitus, pulmonary and cardiovascular disease, exercise and heat intolerance, and orthopedic problems. Surgical risk is higher, and the incidences of postoperative morbidity and mortality increase.
DEVELOPMENT OF OVERWEIGHT CONDITIONS
The fundamental underlying cause of obesity is an imbalance between energy intake and energy expenditure that results in a persistent energy surplus (positive energy balance). Over time, the energy surplus results in weight gain and a change in body composition. The increase in body fat occurs either by an enlargement of fat cell size alone (hypertrophic obesity) or by an increase in both fat cell size and fat cell number (hyperplastic obesity). Pets that develop hyperplastic obesity are generally believed to be difficult to treat and have a poor long-term prognosis. Normal adipocyte hyperplasia occurs during specific critical periods of development. In most species, these periods occur during early growth and occasionally during puberty. 29 Once adulthood is reached, the total number of fat cells does not normally increase further. Overfeeding during adulthood results in an increase in fat cell size, but little or no change in fat cell number. Although conditions of extreme and prolonged overfeeding can result in fat cell hyperplasia in some animals, the majority of cases of adult onset obesity are a result of fat cell hypertrophy alone. 30. and 31.
The body has the capacity to add new adipocytes, but is not able to reduce its existing adipocyte number. This phenomenon, called the “ratchet effect,” indicates that body fat can always increase, but it cannot decrease below a minimum level that is set by the total number of adipocytes and their need to remain lipid-filled. This fact is of importance when considering growth rate and weight gain in young, developing dogs and cats. Data from several studies with laboratory animals show that overnutrition during growth results in increased numbers of fat cells and total body fatness during adulthood. 32. and 33. Superfluous fat cell hyperplasia during the critical periods of adipose tissue growth may produce a long-term stimulus to gain excess weight in the form of excess adipocytes. The greater number of fat cells results in both an increased predisposition toward obesity in adulthood and an increased difficulty in maintaining weight loss when it occurs. Although this effect has not been specifically demonstrated in dogs and cats, it is theorized that persistent overnutrition during development in growing pets may result in both adipocyte hypertrophy and hyperplasia, leading to overweight conditions that are particularly refractory to treatment. The potential for an animal to produce excess numbers of fat cells during specific critical periods illustrates the importance of proper weight control throughout growth.
Similar to humans, dogs and cats may develop overweight conditions gradually, over a period of months or years, in response to a relatively small but prolonged energy imbalance. Conversely, some pets gain weight rapidly, over a period of a few weeks or months. This may occur when energy expenditure decreases significantly following the combined effects of neutering, attainment of mature body size, and reduced activity level, and is not accompanied by a reduction in energy intake. Feeding dogs free choice (ad libitum) may also lead to rapid weight gain when a new food is introduced or when a new dog is added to the home. Both novelty and social facilitation can contribute to a sudden increase in food consumption.
Two stages occur during the development of obesity: the dynamic phase and the static phase. During the initial dynamic phase, an animal consumes more energy than is expended, and the surplus energy is deposited as body fat and, to a lesser degree, as lean body tissue. As the dog or cat gains weight, its resting metabolic rate (RMR) increases proportionately to the increase in lean body mass. Eventually the increased RMR, coupled with the increased energy expenditure that is needed to move a larger body size, offsets the caloric surplus. At this point, zero energy balance is achieved, and the animal stops gaining weight. The static phase of obesity occurs when the animal is no longer gaining weight but achieves energy balance and maintains its overweight condition for a prolonged period of time.
RISK FACTORS FOR OBESITY
Although the problem of obesity appears very simple in terms of energy balance, a multitude of underlying factors can contribute to an animal’s propensity to gain weight and to maintain an overweight body condition. Moreover, the development of obesity in an individual dog or cat can be the result of several separate influencing factors occurring simultaneously (Box 28-2). Factors that may contribute to the development of obesity can be classified as having either an endogenous or an exogenous origin. Endogenous factors include the animal’s age, sex, and reproductive status, hormonal abnormalities, hypothalamic lesions, and genetic predisposition (breed). Exogenous factors include social and environmental influences on food intake, diet composition and palatability, and the pet’s lifestyle (amount and type of exercise). Although most cases of companion animal obesity are a result of overfeeding, underexercising, or a combination of the two, it is important to recognize that each of these two conditions are typically the result of a combination of external and internal influencing factors.
BOX 28-2
| E ndogenous factors | E xogenous factors |
|---|---|
Age, sex, and reproductive status Presence of hormonal abnormalities or hypothalamic lesions Genetic predisposition | Voluntary activity level External influences on food intake Diet composition and palatability Living environment and type of lifestyle |
Inadequate Exercise
A sedentary lifestyle is an important contributor to decreased energy expenditure and to the development of overweight conditions in companion animals. 2. and 34. In today’s society, most dogs are kept as companions and house pets rather than as active, working partners to their human owners. Cats are also experiencing decreased activity levels. A significant proportion of cats lead sedentary, indoor lives rather than having the run of farms and neighborhoods as in the past. Living indoors and apartment dwelling have been identified as risk factors for weight gain in both dogs and cats. 5. and 35. It is postulated that this effect is due to reduced opportunities for outdoor exercise. Additional factors that influence the daily activity level of dogs and cats are breed, temperament, age, reproductive status, and the presence of certain chronic illnesses or developmental disorders. Moreover, a vicious cycle can occur in which an overweight pet becomes increasingly sedentary and reluctant to exercise because of obesity-induced exercise intolerance and mobility problems.
In normal animals experiencing moderate levels of exercise, physical activity contributes about 30% of the body’s total energy expenditure. Decreased voluntary activity results in a direct reduction of this energy expenditure and can also affect a pet’s daily food intake. Research studies have shown that completely sedentary animals actually consume more food and gain more weight than do animals that experience moderate activity levels. 36 It appears that inactivity below a certain level cannot be entirely compensated for by an adequate decrease in food intake. As a result, animals that are maintained at or below this minimum activity level will consume more than their energy needs and will inevitably gain weight.
Factors that influence the amount of energy that is expended for exercise include the type, duration, and frequency of the activity. Other factors that affect the activity level of dogs and cats include breed, temperament, age, reproductive status, and the presence of certain chronic illnesses or developmental disorders.
Increasing Age
Obesity is most common in middle-aged adult dogs and cats. In one study, only 6% of female dogs between 9 and 12 months of age were evaluated as overweight, compared with 40% of mature adults. 37 This same trend is observed in cats. 6. and 7. As an adult animal ages, lean body mass gradually declines, resulting in reduced basal metabolic rate (BMR) and total daily energy needs. The loss of lean body mass is exacerbated if aging is accompanied by a decrease in exercise. The total daily energy needs of an average-size, 7-year-old dog may decrease by as much as 20% when compared with its needs as a young adult. If food intake does not decrease proportionately with decreasing energy needs as an animal ages, weight gain results.
Neutering
The increased incidence of overweight conditions in neutered dogs and cats has been recognized for many years. 38 Recent studies support these observations, showing that neutered adult pets generally weigh more and maintain higher body fat than intact animals of the same breed and size. 39.40. and 41. The underlying cause of these differences is probably a combination of physiological and environmental factors. Veterinarians often encourage clients to castrate or spay their pets shortly before they become sexually mature. As a result, many dogs and cats are neutered between 6 months and 1 year of age. This period corresponds to a natural decrease in activity level and in the animal’s energy needs for growth. If owners are not aware of this change and continue to feed their pet the same amount of food, excess weight gain will result. Because spaying and neutering often occur just before maturity, the change in sexual status may be erroneously blamed for a weight gain that was actually the result of diminished energy needs and excess food intake.
However, there is also a direct effect of reproductive status upon feeding behavior and food intake. Female dogs and cats typically decrease their food intake during estrus, and the cause of this change has been attributed to estrogen. For example, a study with dogs examined the influence of estrus on voluntary food intake in 12 Beagle bitches. 42 Results showed that there was a tendency for females to decrease food consumption during the week that they were in estrus. The authors then examined food intake patterns in ovariohysterectomized and control-operated bitches. Over a period of 90 days, the ovariohysterectomized bitches consumed 20% more food and gained significantly more weight than did the sham-operated controls. The authors attributed the difference in weight gain to an increase in food intake and a decrease in voluntary activity. More recently, a study of ovarioectomized dogs showed that maintenance energy needs decreased while voluntary food intake increased significantly after being spayed. 43 Similar results have been observed in cats. 44 Both male and female cats tend to increase voluntary food intake after neutering and consume more food than intact adults. 45. and 46. Although the exact metabolic mechanism for this change is not known, hormonal alterations following neutering in cats (in addition to the loss of estrogen in females) include increases in plasma concentrations of insulin-like growth factor-1 (IGF-1) and prolactin. 47 It is theorized that increased IGF-1 may stimulate adipocyte proliferation and that persistently high levels of prolactin contribute to the maintenance of adipose tissue and possibly to the dysregulation of glucose metabolism seen in overweight cats. The orexigenic (appetite-stimulating) hormone ghrelin may also be involved in regulation of energy intake in neutered animals.
Finally, an animal’s BMR is affected by neutering. When RMR was measured using respiratory indirect calorimetry in neutered and intact cats, heat coefficients were greater in intact male and female cats than in neutered animals. 39 Intact males had heat coefficients that were 28% higher than those of neutered males, and intact females had heat coefficients that were 33% higher than those of neutered females. When RMR is expressed on the basis of lean body tissue, no difference is seen between intact and neutered animals, suggesting that these differences are caused by the body composition changes seen in neutered animals. Regardless, the dramatic change in BMR can be interpreted to mean that neutered male cats may require 28% fewer calories, and females 33% fewer calories, than their intact counterparts.
Many shelters and veterinarians have adopted the use of early-age neutering (at 8 to 16 weeks of age) because of the benefits to pet population control. Recognition of the safety of these procedures for puppies and kittens led the American Veterinary Medical Association to approve a 1993 resolution supporting the concept of early-age neutering. However, one concern has been the potential of early-age neutering to influence a pet’s tendency to become obese. A study compared metabolic rates and development of obesity in cats that were surgically neutered at 7 weeks of age, surgically neutered at 7 months of age, or left intact. 39 All of the cats in the study were fed ad libitum until they were 2 years of age and were assessed regularly for body condition, metabolic rate, and glucose tolerance. Because body weight alone is not an accurate predictor of obesity, body condition scores were assigned and body mass index was calculated. The body condition scores and body mass indices of the neutered males and females were significantly higher than those in the intact animals, indicating that neutered animals were more obese than intact animals. However, no differences were observed between animals neutered at 7 weeks of age and those neutered at 7 months of age. More recently, an epidemiological study of dogs in the United States found that the frequency of obesity was slightly lower in dogs that were neutered before 5.5 months of age when compared with dogs neutered after 6 months. 48 Together, these results indicate that early-age neutering presents the same level of risk of weight gain as does neutering at the traditional age of 6 to 9 months.
Caloric intake should generally be reduced after neutering to prevent weight gain. Neutering increases a pet’s risk for overweight conditions via several mechanisms. The age that the dog or cat is neutered often corresponds with a natural decrease in the pet’s growth rate and energy needs, which may lead to weight gain. Neutered animals also tend to consume more food and to have a reduced basal metabolic rate, both of which can contribute to an energy surplus if food intake is not controlled.
Genetic Predisposition (Breed)
Certain breeds of dogs reportedly have a disproportionately high incidence of obesity, although the breeds that are identified tend to vary with both the time of the study and region of the world. Early studies in the United Kingdom identified Cocker Spaniels, Labrador Retrievers, Shetland Sheepdogs, and the small Terrier breeds as being predisposed to obesity, while Boxers, German Shepherd Dogs, Fox Terriers, and the sight-hound breeds had a relatively low incidence. 49 Conversely, a study in Germany just a few years earlier reported that German Shepherd Dogs, Boxers, and Poodles were more likely to be overweight. 50 These differences suggest that the popularity of a breed and regional differences in type may influence breed predispositions. A more recent report of dogs in the United States identified Labrador Retrievers and Shetland Sheepdogs, as well as Golden Retrievers, Cocker Spaniels, Dachshunds, Miniature Schnauzers, Springer Spaniels, Chihuahuas, Basset Hounds, and Pugs as most likely to be overweight or obese. 8
Although several environmental factors are also involved, genetically influenced body composition differences may partially explain the higher frequencies of overweight conditions in certain breeds of dogs. For example, it can be theorized that breeds that were developed for physical work and that naturally possess a higher muscle mass to body fat ratio will have a higher BMR than dogs of similar size that have a lower proportion of lean tissue and a higher proportion of body fat. One example is a comparison of Great Danes, bred for protection, with Newfoundlands, bred for cold-water rescue work. Adult Newfoundlands have lower energy requirements than adult Great Danes of similar weight; this difference is speculated to be due to the higher proportion of lean body tissue seen in Great Danes. 51 More research that examines energy differences among dog breeds is necessary to increase understanding of possible predispositions to obesity. Few data are available concerning breed predilections to obesity in pet cats, but two studies have suggested that mixed-breed cats are more likely to be overweight than purebred cats, while another suggests that Manx cats show a higher frequency. 5.6. and 52. As purebred cats become increasingly popular and more breeds are developed, such predilections may become more evident in this species.
Endocrine Disorders
Two endocrine disorders that may influence body weight in companion animals are hypothyroidism and hyperadrenocorticism. Hypothyroidism results in a decreased BMR, which may in turn cause a predisposition for obesity. This disorder is diagnosed when clinical signs are observed and plasma levels of one or both of the thyroid hormone variants thyroxine (T 4) and triiodothyronine (T 3) are found to be below normal. Idiopathic atrophy of the thyroid gland is the most common cause of hypothyroidism in dogs. This disorder occurs most frequently in middle-aged and older dogs, and certain breeds show a higher incidence than the general population. These breeds include Golden Retrievers, Doberman Pinschers, Irish Setters, Boxers, Old English Sheepdogs, Miniature Schnauzers, Airedale Terriers, and some Spaniel breeds. 53 Spayed females are also more likely to develop the disorder than other dogs. 54 Hypothyroidism can occur in cats, but it is much less common and has not been well documented.
Clinical signs of hypothyroidism include lethargy, a dulled mental attitude, and exercise intolerance. 55 Common skin changes include alopecia; the development of a dry, coarse coat; and skin hyperpigmentation. Cold sensitivity and weight gain are clinical signs that result directly from the decreased BMR associated with hypothyroidism. However, only a small percentage of dogs exhibit all of these signs, and when obesity is seen, it is usually moderate. Regardless, an assessment of thyroid hormone levels should always be included in the differential diagnosis of obesity.
Hyperadrenocorticism (Cushing’s syndrome) can also result in increased body size. This disorder is caused by the production of excess corticosteroids by the adrenal cortex, which may be caused by either an adrenal gland or a pituitary gland tumor. It is most common in middle-aged and older dogs, and breed predilections have been observed in Poodles, Dachshunds, Boxers, Brussels Griffons, and Boston Terriers. 56 Cushing’s syndrome can occur in cats, but it is quite rare. The primary clinical signs of this disorder include polyuria, polydipsia, lethargy, hair loss, and the development of a pendulous abdomen. True obesity occurs in approximately 50% of the cases, although the presence of an enlarged abdomen may be perceived to be obesity by some pet owners. Diagnosis is based on adrenal function tests, which will differentiate between Cushing’s-induced obesity and obesity as a result of other causes.
Alterations in Food Intake
Food intake is regulated in all animals by a complex system involving both internal physiological controls and external cues. Internal signals that affect appetite, hunger, and satiety include mechanical stimulation from the gastrointestinal tract; physiological responses to the sight, sound, and smell of food; and changes in plasma concentrations of specific nutrients, hormones, and peptides. External stimuli include factors such as food availability; the presence of other animals; the timing and size of meals; a food’s composition, texture, and palatability; and the pet owner’s beliefs and perceptions. Because owners exert complete control over the feeding of their dogs and cats, external cues that affect food intake are probably most important influences upon intake and the development of obesity in companion animals.
A well-researched external factor is feeding highly palatable foods that induce animals to overconsume. Studies with laboratory animals have shown that when rats are offered a highly palatable diet, they overeat and become obese. 57 This effect has been observed with high-fat diets, calorically dense diets, and “cafeteria” diets, which provide a variety of highly palatable food items. 58 Long-term exposure to highly palatable foods in human subjects also leads to permanent increases in body weight, fat cell size, and fat cell number. Although an endogenous predisposition to obesity and increased efficiency of weight gain may occur in some animals, the largest portion of weight gain observed when animals are fed highly palatable diets is a direct result of overconsumption. Similarly, studies with human subjects have demonstrated that the quantity of food consumed varies directly with its palatability, and palatability does not appear to interact with levels of food deprivation. In other words, if food is perceived to be highly appealing, an individual tends to eat more of it, regardless of his or her initial level of hunger. 59
Palatability is an important diet characteristic that is heavily promoted in the marketing of pet foods. Many owners select a product based upon their perceptions of the food’s appeal and their pet’s acceptance of the diet, rather than on indicators of nutritional adequacy. Semimoist dog foods and treats contain variable amounts of simple sugars and other humectants that contribute to palatability. Wet foods and some premium dry foods are high in fat content. Fat contributes to both the palatability and caloric density of the food. Feeding pets highly palatable foods on an ad libitum basis may contribute to both the development and the maintenance of obesity because many dogs and cats readily overconsume these foods. Similarly, the common practice of feeding a variety of table scraps and other appealing treats to dogs and cats can induce many pets to overeat and gain excessive amounts of weight. For example, a retrospective study of dietary patterns in adult female dogs found that up to 50% of calories supplied to some dogs came from table scraps, particularly for the toy breed dogs. 60 Recent studies report that dogs fed many treats and table scraps or that are fed canned or homemade foods as their primary diet are more likely to be overweight. 8. and 61. Table scraps and some homemade diets that are fed to pets can also contain a high proportion of their calories from fat and thus contribute to a caloric imbalance.
The social setting of meals and pet owners’ behavior and beliefs also influence eating behavior. Many animals increase food intake when consuming food in the presence of other pets. This process is called social facilitation and is usually more pronounced in dogs than in cats. In most dogs, social facilitation causes a moderate increase in food intake and an increased rate of eating. In some, the increase in food intake in response to another animal’s presence can be extreme enough to cause weight gain. The owner’s perceptions can also influence food intake. For example, results of a recent survey of cat owners reported that owners of overweight cats were more likely to interpret their cat’s affiliative behaviors as requests for food than were owners of normal weight cats. 62 Similar results with dog owners have been reported; owners of overweight dogs tended to use food as a way to pacify all types of attention-seeking behaviors, thus using food to reinforce undesirable behaviors and as a “pet baby sitter” to induce the dog to be calm and quiet.
Similarly, meal frequency affects both food intake and metabolic efficiency. An increase in the number of meals per day results in increased energy loss to meal-induced thermogenesis (see Section 2, p. 60). There is also evidence in humans indicating that a decrease in lipogenesis (fat tissue synthesis) occurs when multiple meals are fed, as compared with consuming the same number of calories in only one or two meals. 63 However, if several meals are provided per day, portions must be strictly controlled. Increased feeding frequency often causes increased voluntary intake, thereby offsetting any metabolic benefits of multiple meals.
A final external factor that may be a contributing cause of obesity in companion animals is the nutrient composition of the diet. Nutrient composition affects both the efficiency of nutrient metabolism and the amount of food that is voluntarily consumed. Dietary fat is the primary nutritional factor influencing the development of obesity in humans. When fed ad libitum, high-fat diets lead to weight gain and obesity. 64 Although most animals decrease the volume of intake of a high-fat diet in an attempt to balance energy needs, the greater caloric density of the diet and its increased palatability usually offset this adjustment and result in an overall increase in energy intake. Additionally, the metabolic efficiency of converting dietary fat to body fat for storage is higher than is the efficiency of converting dietary carbohydrate or protein to body fat. For example, a recent study with dogs showed that modifying the diet’s nutrient composition by increasing the proportion of fat by 8% caused a significant increase in abdominal fat deposition in the dogs, even though total caloric intake did not change. 65 Because dietary fat is more efficiently converted to body fat than protein or carbohydrate, if an animal is consuming more than its caloric requirement and if the excess calories are provided by fat, more weight will be gained than if the excess calories are coming from either carbohydrate or protein.
The caloric distribution of fat, carbohydrate, and protein is very important in determining a diet’s potential contribution to weight imbalance in dogs and cats. As the percentage of metabolizable energy (ME) calories from fat increases in a pet food, the ability of the diet to meet the high energy demands of a hard-working dog also increases. However, if this diet is fed to a dog that does not need it, weight gain may occur if intake is not strictly monitored. Likewise, a food that contains a lower percentage of ME from fat will aid in weight loss or in the maintenance of normal body weight in a sedentary animal. The selection of a pet food should therefore always match the proportion of ME contributed by fat to the animal’s lifestyle and activity level (Figure 28-1).
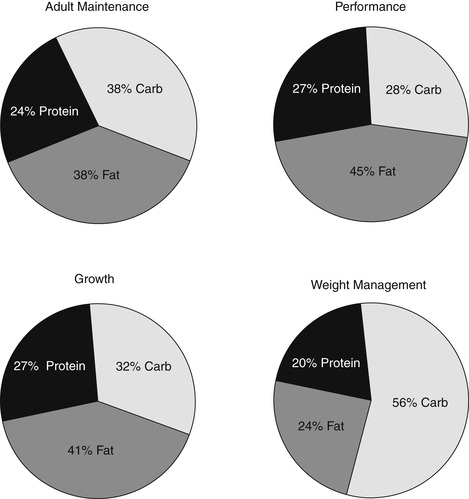 |
| Figure 28-1 |
Important factors that can contribute to the development of obesity in dogs and cats include diet palatability, food composition and texture, and the timing and environment of meals. Just like their human counterparts, animals (especially dogs) tend to eat more, regardless of their initial level of hunger, when the food is highly palatable and presented in a social setting. More weight is gained when excess calories come from dietary fat than from protein or carbohydrate.
In recent years, the internal controls of hunger and satiety and their roles in obesity have received a great deal of attention (see Chapter 9, pp. 61-63). A wide range of neuroendocrine factors are involved in hunger and satiety. The functions and interactions of these compounds are complex and are influenced by numerous internal and external controls. Two peptides that may be important in the control of food intake in overweight animals are ghrelin and leptin. Ghrelin (i.e., growth hormone–releasing factor) is an enteric, orexigenic (appetite-stimulating) peptide. It stimulates the secretion of growth hormone and has been shown to increase appetite and food intake in human subjects and rats. 66 Plasma concentrations of ghrelin increase during the fasting state and decease postprandially. Recent studies have shown that obese dogs, like overweight human subjects, have lower circulating plasma ghrelin concentrations than do nonobese animals. 67 There is also evidence that obese subjects that have lost weight have higher plasma ghrelin levels. 68 It is postulated that this increase may affect appetite and may be an important factor in the common tendency for previously overweight individuals to regain some or all of their lost weight.
Leptin is a cytokine produced by adipocytes that acts as a regulator of energy balance in the body. Its secretion is regulated by a variety of neuroendocrine factors, including insulin, glucocorticoids, and catecholamines. Circulating levels of leptin are directly proportional to the quantity of fat cells in the body; obese animals have chronically elevated plasma leptin. 69 Recent studies with dogs show that plasma leptin concentrations are highly correlated with body fat and its measure can be used as a quantitative marker for adiposity. 70 This relationship has also been demonstrated in cats, as well as a relationship between plasma leptin concentrations and insulin resistance, a finding that may be important for understanding factors that affect the development of diabetes in overweight cats. While leptin appears to increase energy expenditure in normal weight individuals and reduces appetite when injected, its effects in obese dogs and cats and its relationship to the development and maintenance of overweight conditions are not completely clear. There is some research suggesting that vitamin A intake influences leptin by normalizing serum leptin levels during weight gain, which may contribute to reduced adiposity. 71 Other internal regulators of food intake that require more study include adiponectin, a cytokine that acts in synergy with leptin; tumor necrosis factor-alpha (TNF-α), a proinflammatory molecule that may be involved in insulin resistance; and a variety of uncoupling proteins (UCPs) that are located in the mitochondrial membrane and function to uncouple the respiration of adenosine triphosphate (ATP) synthesis and which may be involved in dietary thermogenesis. 72
DIAGNOSIS
The diagnosis of obesity in companion animals should always include a veterinary examination for the presence of edema, ascites, hypothyroidism, hyperadrenocorticism, and diabetes mellitus. After these diseases have been ruled out, a comparison of the pet’s current body weight with previous weight measurements or with the pet’s weight shortly after reaching adulthood may be indicative of abnormal weight gain. Body weight in dogs and cats correlates moderately with body fat mass. Therefore recording a pet’s body weight regularly provides a sensitive indicator of changes in body condition. 73 In some cases involving purebred dogs and cats, a comparison of the pet’s body weight with the weights suggested by the breed’s standard may also be a useful guideline for determining ideal body weight (TABLE 28-1 and TABLE 28-2; also see Appendix 3, pp. 525-528).
| B reed | M ale (lb) | F emale (lb) |
|---|---|---|
| Basset Hound | 65-75 | 50-65 |
| Beagle (13″) | 13-18 | 13-16 |
| Beagle (15″) | 17-22 | 15-20 |
| Boxer | 55-70 | 50-60 |
| Chihuahua | 2-6 | 2-6 |
| Chow Chow | 45-50 | 40-50 |
| Cocker Spaniel | 25-30 | 20-25 |
| Collie | 65-75 | 50-65 |
| Dachshund, Miniature | 8-10 | 8-10 |
| Dachshund, Standard | 16-22 | 16-22 |
| Dalmatian | 50-65 | 45-55 |
| Doberman Pinscher | 65-80 | 55-70 |
| English Springer Spaniel | 49-55 | 40-45 |
| German Shepherd | 75-90 | 65-80 |
| Golden Retriever | 65-75 | 55-65 |
| Labrador Retriever | 65-80 | 55-70 |
| Maltese | 4-6 | 4-6 |
| Miniature Schnauzer | 16-18 | 12-16 |
| Pekingese | 10-14 | 10-14 |
| Pomeranian | 4-7 | 3-5 |
| Poodle, Standard | 50-60 | 45-55 |
| Poodle, Miniature | 17-20 | 15-20 |
| Poodle, Toy | 7-10 | 7-10 |
| Rottweiler | 80-95 | 70-85 |
| Shetland Sheepdog | 16-22 | 14-18 |
| Shih Tzu | 12-17 | 10-15 |
| Siberian Husky | 45-60 | 35-50 |
| Yorkshire Terrier | 4-7 | 3-6 |
| B reed | M ale (lb) | F emale (lb) |
|---|---|---|
| Abyssinian | 7-10 | 6-8 |
| American Shorthair | 10-15 | 8-12 |
| Birman | 9-15 | 6-10 |
| British Shorthair | 12-18 | 9-15 |
| Burmese | 8-12 | 6-10 |
| Cornish Rex | 6-9 | 5-7 |
| Devon Rex | 8-10 | 5-8 |
| Egyptian Mau | 10-14 | 6-10 |
| Exotic | 7-14 | 6-10 |
| Maine Coon | 14-20 | 9-12 |
| Norwegian Forest Cat | 10-16 | 8-12 |
| Ocicat | 10-15 | 7-12 |
| Persian | 9-14 | 7-11 |
| Ragdoll | 12-20 | 8-15 |
| Russian Blue | 7-11 | 5-8 |
| Scottish Fold | 9-13 | 6-9 |
| Siamese | 11-15 | 8-12 |
| Sphynx | 8-12 | 6-9 |
| Tonkinese | 8-12 | 6-8 |
Estimating percent body fat is the most accurate method of diagnosing obesity. Ultrasound provides a noninvasive, rapid method for estimating subcutaneous fat, but it is does not provide an estimate of whole body fat and is not practical in most clinical settings. 74 Likewise, estimates of lean and fat tissue in the body using the heavy isotope deuterium oxide is noninvasive and accurate, but requires sequential blood sampling and access to mass spectrometry equipment. 75 Dual energy x-ray absorptiometry (DEXA) has been shown to provide a very accurate measurement of total body fat and lean body mass and is frequently the method of choice in clinical trials. 76 This procedure has been used extensively in research settings to determine the body composition of many species, including dogs and cats. 77 However, DEXA is neither practical nor economical for use by most practicing veterinarians. Finally, bioelectrical impedance analysis (BIA) has been examined as a rapid and noninvasive clinical method for determining quantity of lean and fat tissue in pets. 78. and 79. BIA involves applying a series of electrodes to the surface of the animal’s body and measuring degree of conductance and capacitrance when a low-level electrical current is applied. Because the body’s fat free mass (FFM) contains more water than does its adipose tissue, lean tissue has a higher conducting volume than fat tissue. Conversely, fat cells cause greater impedance to the passage of current. BIA provides a comparative ratio of these two properties, and allows calculation of a value of FFM and fat mass. Although commercially available BIA systems are available to practitioners, the recorded values are influenced by a number of factors, including but not limited to the pet’s size, age, posture, hydration status, and fed or fasted state. Although BIA has been shown to be a reliable measure of body composition when all of these influencing factors are controlled, its sensitivity to environmental conditions and need for standardization make it currently impractical for most veterinary clinic settings. 80
The most practical method for assessing excess body fat and obesity in dogs and cats is the use of morphometric techniques that combine the measurement and evaluation of visible body features and palpation of regions of the body that correspond to major adipose deposits. For dogs and cats, visual assessment is followed by palpation of the thickness of tissue overlying the rib cage, lumbar area, and tail base, and the thickness along the ventral abdomen. If a dog or cat is too thin, the ribs will be easily seen. An animal of normal weight will have barely visible ribs that can be easily felt when palpated. An overweight animal’s ribs will not be visible and an overlying layer of fat can be felt. The pet is diagnosed as grossly obese if the ribs cannot be felt at all.
For practicing veterinarians and pet owners, visual assessment of dogs and cats can be standardized by using a body condition scoring (BCS) system. Several systems are available to practitioners and pet owners, all of which involve subjective ranking of body composition based upon visual assessment and palpation (Figure 28-2 and Figure 28-3). The two most common systems use either five points (a score of 3 corresponds to ideal body condition) or nine points (a score of 5 corresponds to ideal body condition). Because the five-point system typically includes half-point assessments, the two scales are in practice very similar. Clinical trials have shown that body scoring systems provide a highly reliable method for the diagnosis of obesity and are predictive of percent body fat. 81.82. and 83. Comparisons of body composition data collected using DEXA with assessments of body condition using a nine-point BCS revealed significant and positive correlations between body condition scores and percent body fat in both dogs and cats. Interestingly, although the predictive value of the scoring system was the same for both male and female pets, females of both species had a higher percentage of body fat than males that were assigned the same body condition score. 82. and 83. Two limitations of BCS systems are that their subjectivity can lead to wide interobserver variation and that some training in morphometric assessments may be needed. Recently, a seven-point system has been developed that provides an easy-to-use flow-chart and set of diagrams for pet owners. 84 However, this system still requires validation studies to determine if it effectively identifies overweight and obese pets.
 |
| Figure 28-2 (Copyright © Procter & Gamble Co., Cincinnati, Ohio, 2009.) |
 |
| Figure 28-3 (Copyright © Procter & Gamble Co., Cincinnati, Ohio, 2009.) |
BCS involves the assessment of several different areas of the body. Dogs and cats that are at their ideal body weight should have an hourglass shape when viewed from above, showing an observable waist behind the ribs (see Figure 28-2 and Figure 28-3). In heavily coated animals, the waist should be easily palpated beneath the pet’s hair coat. The loss of a waist as a result of excess fat between the muscles of the abdominal wall and the presence of a pendulous abdomen as a result of fat accumulation in intraabdominal sites are both indicative of excess body fat. Dogs have a tendency to develop fat deposits over the thorax and spine and around the base of the tail, while cats often accumulate fat just anterior to the inguinal region. In addition, overweight cats develop folds of skin and underlying fat in the flank area. Subjective evaluation of the animal’s gait, exercise tolerance, and overall appearance can also be used to support a diagnosis of obesity.
The development of visual body condition assessment tools is also of benefit to pet owners. Veterinary practitioners can use illustrative charts to teach clients to monitor their pets’ weights and body conditions. Standardized visual aids are helpful because an owner’s perception of his pet’s weight is often inaccurate. A survey of dog owners found that when clients were asked to identify whether their dog was overweight, underweight, or at an appropriate body weight, approximately 30% to 40% of owners of overweight dogs felt their dog was at an appropriate body weight. 83 Similarly, owners of overweight cats tended to underestimate their cat’s score on a standard body condition scale. 85 Interestingly, when cat owners and veterinarians were asked to select overweight cats from a series of illustrations of cats’ silhouettes, owners and veterinarians were in close agreement as to which animals were overweight. Because some owners may be unable or reluctant to recognize weight gain or obesity in their own pet, the use of body condition charts may be helpful to veterinarians as a tool to teach about ideal body weight and to convince some owners of their pet’s overweight condition.
Comparison of a pet’s current body weight to an estimated ideal body weight, visual assessment and the use of body condition scoring are the most practical and reliable means of diagnosing obesity. Dogs and cats at ideal body weight should have an hourglass shape when viewed from above, showing an observable and easily palpable waist. Dogs have a tendency to develop fat deposits over the thorax and spine and around the base of the tail, while cats often accumulate fat just anterior to the inguinal region. Subjective evaluation gait, exercise tolerance, and overall appearance can also be used to support a diagnosis of obesity.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


