4 Principles of dermatological therapeutics
Anti-infective pharmaceuticals used in dermatology
Principles of antibiotic therapy in skin disease – the use of antimicrobials in wound management and skin disease
Dermatological shampoos/washes
Principles of immunotherapy in dermatology
Principles of dermatological chemotherapy and cancer medicine
Principles of surgical treatment of skin disease
The treatment of skin disease provides many challenges for the clinician. These include the type of disease and the practicality of many of the normal therapeutic options when used in such a large animal. As with all treatment protocols, in selecting a treatment method the clinician should consider its likely benefits and any possible undesirable consequences; most medications that have at least one benefit (usually the therapeutic effect) will also have at least some potential for harm (the side- or unwanted effects). Thus, a surgical option might present itself, but the resulting wound might be impossible to close, or the resultant scar might cause significant functional problems that may be more serious than the condition itself. For example, a sarcoid in the upper eyelid or on the coronary band would be extremely difficult to remove surgically or treat with a necrotizing topical cream without some functional deficit. The treatment in such cases may be far worse than the condition. A much more careful choice of treatment would almost certainly have less secondary effect (Fig. 4.1). Of course, the pressures of practice often mean that owners want a ‘cheap, quick fix’ and are often intolerant of a more gentle approach or a more expensive option.
There is no evolutionary advantage in disease and so in normal circumstances the natural reparative mechanisms are deployed to attempt repair. Notwithstanding the therapeutic benefits of drugs they cannot substitute for a healthy immune and defence system. A healthy body is less inclined to disease and more inclined to heal well. The importance of a healthy diet and lifestyle is often overlooked in the management of disease. Simple nutritional management can help significantly in maintaining a healthy skin and hair, preventing disease, and supporting recovery from disease of any type. The benefits of omega oils and vitamins and minerals on skin health are well known; a healthy well-balanced diet is usually reflected in a healthy skin that is highly resistant to opportunistic pathogens. Nutritionally deprived and immunocompromised horses will always have greater susceptibility to disease and more difficulty with any therapy than healthy horses fed a healthy diet. The concept of nutritional deficiency and the requirement for supplemental vitamins, minerals and oils, etc. has become almost standard practice in horse management. The truth is, however, that the vast majority of healthy horses are very tolerant of minor and short-term deficiencies and will remain healthy even in the face of general or specific nutritional deprivation. Over-enthusiastic supplementation with any nutrient component can result in significant imbalances and this applies as much to the skin as to other organs and structures. For example, selenium supplementation is common but overdoses can lead to severe and even life-threatening disease. Unfortunately in many parts of the world, vitamin and mineral supplements, herbal remedies and feed additives that purport to ‘enhance’ immunity and resistance to disease are advertised in such a way that most horse owners are drawn to their use regardless of the actual value and regardless of the possibility of harm rather than benefit. Indeed most such supplements are simply a waste of money. Some can be harmful rather than beneficial.
1. The diagnosis may be wrong and therefore the selected treatment is inappropriate. In dermatology this is a relatively common event because many of the clinical symptoms are representative of several very different disease conditions.
2. The diagnosis may be correct but the wrong treatment is used.
3. The diagnosis is right and the treatment selected is correct but it is being applied wrongly. As most dermatological therapy relies heavily on owner and patient compliance there is scope for further problems to develop. It is vital that data sheets for all the drugs are read and re-read to ensure that the best possible information is available regarding side-effects and drug conflicts.
4. The condition is untreatable. Under these conditions treatment is palliative at best but a worsening of the disease situation can occur if inappropriate drugs are used. It may in some circumstances serve the animal best if nothing is done at all.
5. The patient is not cooperative. Some treatments are painful or uncomfortable and application can either be compromised or in some cases is impossible. Even oral medications with steroids or antibiotics can be problematic. Many drugs work best when short intervals between doses/applications are made but the ideal circumstances may simply not be possible.
6. There is failure of owner compliance. The owner may be unable or unwilling to undertake the treatment for any one of a number of valid and invalid reasons. Sometimes there is a perception that the drugs being offered are ‘harmful’. This applies in particular nowadays to corticosteroids. Although there is no scientific evidence that laminitis is an inevitable consequence of corticosteroid administration, a number of high profile cases have given this misconception some credence. Recent research confirms that the risks are very low but that certain types of case and certain types of horse may be more liable to complications.
Generic drug names are used throughout in this text.
Anti-infective pharmaceuticals used in dermatology
Antiseptics/disinfectants
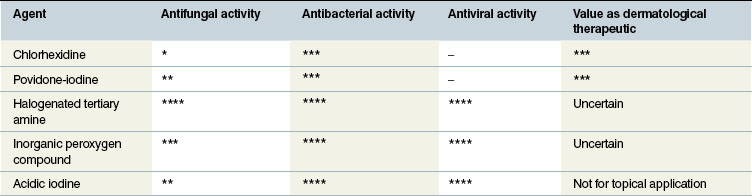
Povidone-iodine
A non-irritating, non-staining, iodophor which comprises iodine solubilized by surface-active agents. It has a broad spectrum of activity with virucidal, bactericidal (and sporicidal) and fungicidal effects which are not affected by blood, serum, necrotic tissue or pus. The rapid bactericidal effects are better at lower dilution – higher concentrations result in reduced bactericidal effects and greater tissue toxicity. The mild tissue toxicity effects are minimized by dilution to less than 0.05%. Solutions of 5% or more actively inhibit leukocyte migration into wounds, while very weak solutions appear to encourage neutrophil and monocyte migration. One per cent solutions cause death of fibroblasts but 0.01% has no detectable harmful effect on these cells.
Fumigation sterilization of tack and equipment using formaldehyde gas
Equipment required
Precautions
• All procedures should be carried out in the open air under cover (ventilation is extremely important as the gas is very toxic).
• Ensure that bags are airtight.
• Avoid contact with either permanganate or formalin solution. It is advisable to wear a mask, gloves (preferably up to the elbow), rubber boots and protective waterproof clothing.
Method
1. Place the articles to be sterilized in a large plastic bag.
2. Place a plastic bowl with potassium permanganate crystals (Condy’s crystals) into the bag.
3. Pour the 40% formalin solution onto the crystals.
5. Leave undisturbed for at least 12 hours.
6. Open the bag in fresh air without breathing fumes – plastic bags can be easily split open and left in the open air.
7. Leave the bag open for 12 hours before handling any equipment inside it.
• Although this is an excellent method for disinfecting equipment it can be a dangerous procedure.
• Formaldehyde gas can be seriously toxic to humans if inhaled in quantity or on repeated occasions.
• The strong formalin solution is also toxic and should not be touched.
• Proper protective measures must be taken at all times to ensure that no human hazard is present.
Antiviral agents
There are few if any specific antiviral agents currently in use in horses. In theory at least some of the new antiviral drugs (such as aciclovir, trifluoridine, ganciclovir and vidarabine) would be expected to have significant benefits on virally induced skin disease and in particular the herpesvirus conditions. Only aciclovir has any equine reputation because the others are both expensive and largely unavailable in the amounts required for horses (both topically or systemically). Aciclovir is currently limited to treatment of putative herpetiform keratitis. Its action is specifically against herpesviruses.
Principles of antibiotic therapy in skin disease – the use of antimicrobials in wound management and skin disease
Potential side-effects include:
• anaphylactic/hypersensitivity reactions including cutaneous urticaria
• delayed drug-related skin eruptions which may occur up to 6 months after administration
• immunologically-mediated systemic effects
• overgrowth of other bacteria or fungal species which may be pathogens that are normally held in check by the commensal microorganisms, or opportunistic pathogens that take advantage of local immunosuppression
• gastrointestinal disturbances deriving from alteration of the delicate large bowel bacterial flora.
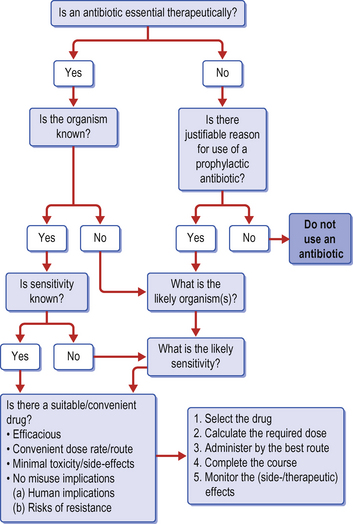
1. Is an antibacterial agent really indicated either prophylactically or therapeutically?
2. Should a culture and sensitivity test be performed before embarking on the medication and is there sufficient justification for the inevitable consequent delay in institution of the therapy?
3. Is the chosen agent appropriate for the case and the suspected (or confirmed) condition?
4. What are the correct dose, dosing interval and route of administration?
5. Is its use likely to result in early resistance and are there measures which can be taken to minimize this risk?
6. Is the duration of therapy appropriate and should any monitoring procedures be performed (to assess either resistance of pathogen or harmful side-effects on the patient)?
Therapeutic antibiotic medication
Antibiotics are required when suspected or confirmed infection is present. Confirmation is obtained by culture of swabs taken from the site (see above) and subsequent culture, Gram stain and antimicrobial sensitivity test. In the absence of confirmation of an infection, clinical signs of inflammation (heat, pain, swelling, redness) or lack of response to supportive therapy are sufficient to warrant the use of antibiotics. As cultures often take several days to perform, it is often wise to pre-empt the findings by administration of a suitable and logical antibiotic and adjust the drug if necessary when results become available. A clinical judgement of the likely infectious organisms based on experience can usually be made.
• What organism(s) is most likely to be present given the history and cause of the disease?
• What is the likely antibiotic sensitivity profile of the suspected bacteria?
The reasons for failure of a detectable therapeutic response to antibiotic therapy include:
1. inappropriate drug selection for the species of bacteria involved
2. inappropriate dose and dose intervals so that the drug never reaches a therapeutic concentration
3. inappropriate drug delivery dynamics/administration route
4. resistant (strains of) bacteria
5. inability of the drug to reach the site due to a compromise in the blood supply or the presence of fibrous tissue or other local problems
6. inactivation of the antibiotic due to enzymatic inactivation or an unsuitable pH in the tissue. Local factors such as the presence of destructive enzymes, acidic pH, low oxygen tension and intracellular organisms can have a marked effect upon the in-vivo efficacy of the drug, even when the in-vitro sensitivity is established.
Ideally, choice of antimicrobial agent will be based on culture and sensitivity results. However, the decision regarding immediate antibiotic administration must invariably be based on clinical judgement (Fig. 4.2). This requires an assessment of the location and nature of the condition, and more importantly knowledge of the organism(s) most likely to be present. For instance, a kick wound may have a significant infection with Escherichia coli resulting from direct contamination but could also have pathological skin contaminant bacteria such as Staphylococcus aureus, S. intermedius or S. epidermidis. Once the potentially useful antibiotics are selected, the final choice may be based on the route or frequency of administration, the location of the infection, the pharmacokinetics of the drug, the cost of the drug, and the clinician’s preference.
When should antibiotics be used in skin disease?
The number and type of bacteria in a wound or skin lesion are the major considerations governing wound contamination and infection (Spurlock & Hanie 1989). These can be further complicated by tissue trauma and necrosis. Impairment of the local blood supply and hence lowered delivery of immunoglobulins and leukocytes as well as the general condition of the horse are also important considerations. Old, debilitated horses often have poor responses to skin injury or insult. The use of systemic glucocorticoids also increases the risk of more severe bacterial invasion of the tissues.
How can the best (most effective) and most convenient antibiotic be selected?
Various factors need to be considered when selecting an antimicrobial drug.
• The sensitivity of the bacteria involved (or suspected). The in-vitro sensitivity to a given antibiotic does not guarantee in-vivo efficacy. Lack of response may be related to tissue concentrations of the active agent as well as the extent to which the organism is in contact with the drug. Thus, some organisms, for example Rhodococcus equi, are largely intracellular and may have a lipid capsule which inhibits antibiotic effects. Positive identification of the organism and its antibiotic sensitivity provide the best selection criteria. However, some laboratories include antibiotics that are either contraindicated in horses or are not available in suitable forms in sensitivity tests and others do not test specifically for those that are available and in common usage; results should therefore be interpreted with some care.
• Antibiotic blood levels affect the tissue levels because passive diffusion appears to be the most likely method of delivery of most antimicrobials. Efficacy is then subject to the activity in the presence of pus, tissue enzymes, acidity/alkalinity (pH) and fibrinous exudate, each of which can influence the clinical response.
• The relative benefits of bactericidal or bacteriostatic effects are important. Bactericidal antibiotics such as penicillin are usually preferred for skin diseases and wounds. In these cases there is then less reliance upon the body’s own defence mechanisms. However, many bactericidal antibiotics are not effective, or are contraindicated in horses, or fail to reach the desired concentration in the desired site. This promotes the development of antibiotic resistance within the wound. Bacteriostatic antibiotics such as oxytetracycline rely ultimately on the natural defence mechanisms to remove the inhibited bacteria so there has to be a healthy cellular immune response.
• Safety and freedom from side-effects and idiosyncratic reactions are obviously important. Unpredictable (idiosyncratic) side-effects such as urticaria, anaphylaxis, etc. are entirely possible with any antibiotic but for the most part are commonest with the penicillin groups. Other drugs (such as lincomycin) have more predictable harmful side-effects (such as diarrhoea). Drug eruptions involving dermatological and systemic effects (such as immune-mediated vasculitis, systemic lupus erythematosus-like syndrome and thrombocytopenia) are less predictable and may follow the administration of any drug (although antibiotics probably have the greatest tendency to induce this) over a period of weeks or months following administration. Drug eruption is a rare but important skin condition in horses (see p. 288) and antibiotics have been implicated.
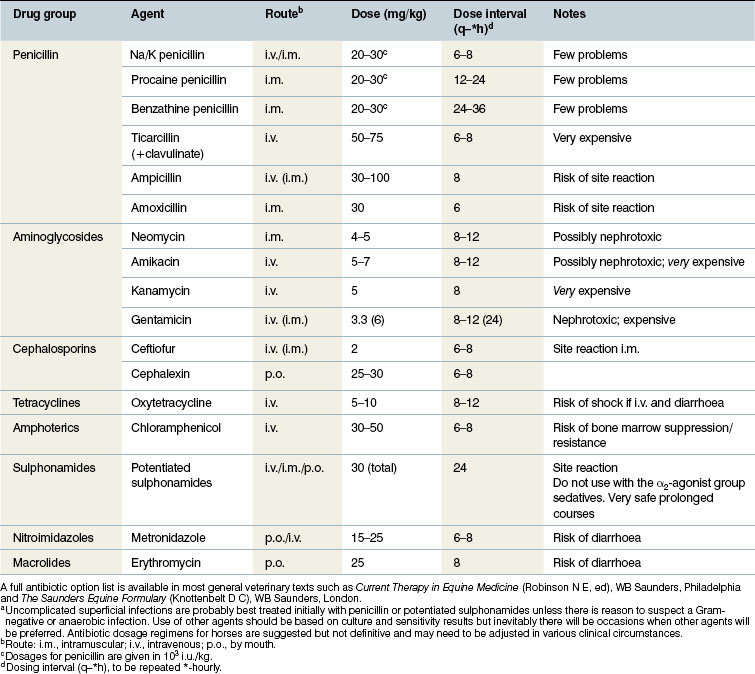
• The cost of the drug will also influence the choice. Many of the most effective, dermatologically active antibiotics are very expensive and involve regimens of treatment which make their use impractical. The cheapest drugs are usually basic penicillin, oxytetracycline and the potentiated sulphonamides. Fortunately, these are commonly sufficiently effective to make them valuable in the treatment of skin diseases.
Route of administration
The standard options available for administration of antibiotics to horses are:
Intravenous administration provides a rapid (almost instantaneous), high plasma concentration. This route often requires frequent dosing and may need special training to administer; it is usually therefore expensive. The need for special training can be avoided by the placement of an intravenous catheter; however, this may not be feasible outside the hospital and in any case catheter management can be difficult. It has been established that because its efficacy is concentration dependent rather than time dependent, gentamicin can be administered effectively using 6.6 mg/kg once daily (Magdesian et al 1994) rather than 2.2 mg/kg three times daily and this clearly makes its administration more economical (Geor 1997).
Possible side-effects of intravenous drug administration include:
• jugular vein thrombosis/thrombophlebitis
• accidental intra-arterial injection
• accidental extravascular injection with local tissue effects on skin, connective tissue and other structures in the vicinity of the vein, for example the vago-sympathetic trunk
• bolus effects from over-rapid injection of a drug which might have effects on the heart or brain
Unwanted side-effects are rare but include:
• abscessation (either sterile or infected)
• damage to adjacent structures such as nerves, vessels and joints
• anaphylaxis (this is fortunately very rare but the risk is always present whenever a drug is administered parenterally).
Duration of therapy
Table 4.2 shows the commonly used antimicrobial agents and gives a summary of the regimens for their use. It is important to remember that none of these can be definitive and the clinician must make a careful assessment of the case as above before embarking on any therapeutic course. Furthermore, the clinician should be satisfied as to the safety and relevance of the regimen to be used.
Antifungal agents
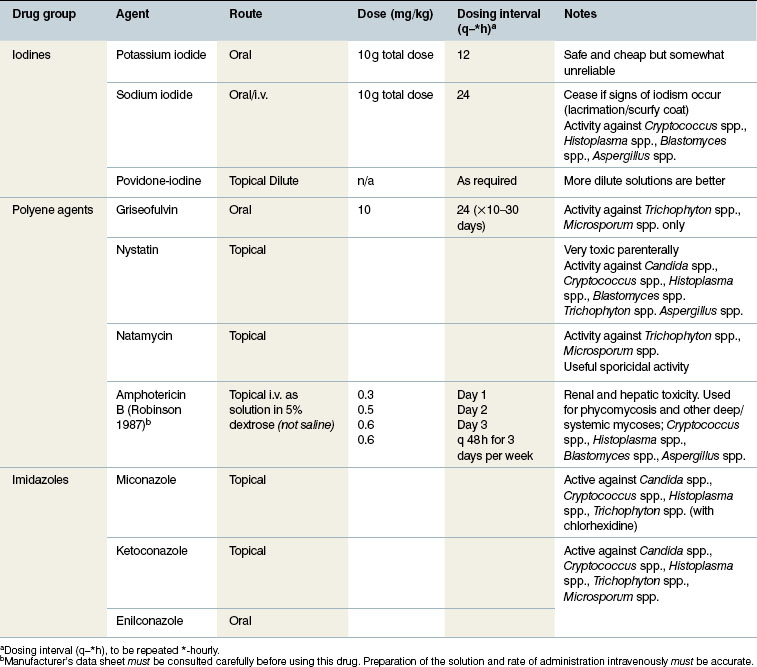
Sodium iodide and potassium iodide are underutilized, effective and safe antibacterial and antifungal agents when administered by intravenous and oral routes, respectively; the potassium salt must not be administered by intravenous injection. A dose of 10 g per 450 kg bodyweight once daily given in the feed has a detectable if mild broad-spectrum antibacterial and antifungal effect against Aspergillus spp. It is convenient and accurate to use a twice-daily dose of 25 mL of an aqueous solution of 160 g of potassium iodide dissolved in 400 mL of water. The solution can be mixed easily with the food and is generally totally unnoticed by the horse. Lesser doses can be effective also. The drug can be continued until obvious signs of iodism develop (lacrimation, a sticky greenish naso-ocular discharge and later a scurfy, flaky skin are the major signs in most cases). If these signs develop, the compound can be withdrawn for 7 days and re-administered if needed. The effects are slow to develop and dramatic responses are unlikely in any deep-seated bacterial or fungal infection.
Griseofulvin is a microsized-particle antifungal agent used in the treatment of superficial mycosis (such as dermatophytosis/ringworm). The drug has been used for many years and has gained a good reputation but recent results are not as satisfying. Reports of the efficacy of griseofulvin are anecdotal – no extensive trials have been reported in support of this. This may be due to resistance or another factor not yet established. It is a tasteless powder which is well tolerated for prolonged courses: courses of up to 30–40 days may be required in some cases. There is no detectable effect against Aspergillus spp. or other deep mycoses. It is potentially teratogenic and so should not be used during pregnancy.
A commercial mixture of 2% miconazole with 2% chlorhexidine has been shown to be useful as a whole-body antifungal shampoo and an environmental antifungal agent (Paterson 1997).
Enilconazole is very effective when applied topically to ringworm-infected horses (Paterson 1997). It is applied directly to the lesions and the surrounding skin every 3 days for three or four applications. Alternatively, the horse can be sprayed with the diluted solution. The drug is not absorbed from the skin and has no significant toxicity apart from the irritant effect of very strong solutions incorrectly applied.
Potassium monopersulphate can be used to control environmental fungal infections (Paterson 1997) and many of the available disinfectants (see above) have strong antifungal and sporicidal effects.
The common antifungal drugs and agents are shown in Table 4.3.
Ectoparasiticides
Prevention of exposure is often the pragmatic approach to insect challenge (Fig. 4.3).
Chlorinated hydrocarbons
In most countries production and use of these compounds is prohibited due to toxicity and residue problems. Gamma benzene hexachloride and bromocyclen are probably the last of these compounds which are available in some countries. There is little doubt that the compounds were effective and that their loss has a significant implication in the treatment of parasitic infections. Some human anti-parasitic washes and shampoos, however, still have the chemicals and some of these may be safely used on horses where such an approach is legally and ethically acceptable.
Organophosphates
Commonly used insecticidal compounds which have been used for horses include dichlorvos, diazinon, malathion, phosmet and chlorpyrifos. Phoxim (Sebacil, Bayer, Germany) is still available in some parts of Europe and can be an effective compound on horses. These compounds are potentially toxic if used improperly and they may have damaging environmental effects also. All have been used topically in horses (usually as washes or dips but occasionally as pour-on compounds) and have good anti-ectoparasitic effects. Most of the compounds have a potent anticholinesterase activity but vary in their toxicity. Application must be strictly according to the manufacturer’s instructions. Clinical signs of toxicity can develop rapidly following their use, particularly in overtired/exhausted horses or in hot humid weather or as a result of over-frequent dipping or from over-strength applications. Signs of toxicity include salivation, trembling or convulsions, constricted pupils, diarrhoea and collapse. Simultaneous use of other organophosphates (such as dichlorvos-based anthelmintics) makes toxicity even more likely. Treatment of the toxic signs includes administration of atropine sulphate by injection and control of convulsions.
Natural insecticidal compounds
As the more effective drugs are discontinued the value of these compounds may need to be reviewed.
Synthetic pyrethroids
Commonly used compounds include deltamethrin, permethrin, cypermethrin and fenvalerate. These compounds have almost no known toxicity at any dose and so are extremely safe. They are frequently used topically against skin parasites. Piperonyl butoxide is commonly added to preparations to enhance the activity. They have a higher efficacy and longer duration than the natural pyrethrins which they have largely replaced. The synthetic pyrethroids are particularly useful as repellents for Culicoides spp. midges and a few species of fly but must be applied at least weekly and immediately after rain wetting in order to achieve continuous protection. Cypermethrin and permethrin have been used as repellents in fly fringes and tags for attachment to head collars and the mane and tail hair (Fig. 4.4).
Other agents
1. Selenium sulphide (1%). A shampoo for the treatment of seborrhoea in pet animals, it has been used for the treatment of lice in particular, in horses (Paterson & Orrell 1995). It is claimed that the shampoo also reduces the secondary seborrhoea which is characteristic of lice infestation.
2. Macrocyclic lactones (avermectin compounds). These are commonly used for the treatment of helminth and bot (Gasterophilus spp.) parasites. Ivermectin and to a lesser extent moxidectin administered orally has been shown to have an effect in reducing the extent of egg laying by lice (Werneckiella (Damalinia) equi and Haematopinus asini) and mites (Chorioptes spp., Sarcoptes spp. and Psoroptes spp.) and is anecdotally reported to have some effect against the adult stages also, but the effects are unpredictable and generally it seems that it is unlikely to eliminate the parasites (Barth & Sutherland 1983). In some parts of the world injectable forms of doramectin are preferred as a means of controlling ectoparasites, especially those that are blood or lymph feeders (ticks, sucking lice and lymph-feeding mites).
3. Insect growth regulators. Most of these compounds have been developed for the control of fleas in small animals. They specifically target chitin, which is a major component of the insect exoskeleton and which does not exist at all in the mammalian body. Currently available insect growth regulators include benzoyl phenylurea derivatives and pyrimidine derivatives. Whilst these have been used on farm animals there is no study on their general application to horses. Possibly the best value is in their use in stables infested with fleas of various types.
4. Lime sulphur. Sulphur and compounds that contain it can be effective parasiticides and are relatively non-toxic and have few environmental concerns. Lime sulphur is a cheap and readily available wash in some countries and has been shown to be effective in horses for controlling surface mites and lice in particular. Sulphur washes of this type have a foul smell that lingers for a long time and they tend to stain white hair quite badly. Therefore they are largely unpopular with owners but are worth trying when other materials are either ineffective or unavailable.
Corticosteroids
General considerations for the use of corticosteroids
It is important when selecting a corticosteroid to consider its specific pharmacokinetics. For example, prednisone is widely used in other species but it cannot be converted into an effective metabolite in horses. There is little evidence for its efficacy and it probably has no effect beyond placebo in most cases – it therefore has no material value in dermatological therapy. The drug distribution of methylprednisolone acetate is such that its value in dermatology is also very limited. Its supposed depot formulation results in poor volume distribution and unpredictable blood concentrations. Triamcinolone is possibly the compound that is associated with most of the largely irrational fear of corticosteroids in horses. Evidence-based medicine suggests that it is not as liable to induce laminitis as it was previously thought (Cornelisse & Robinson 2004, McCluskey & Kavenagh 2004). As might be expected, there are limits to the efficacy of any drug and potent glucocorticoids are no exception. Maximal doses should be accepted and not exceeded. Furthermore, there may be circumstances when the pharmacokinetics are impaired or altered and then the dangers may be far greater. No drug should ever be administered without due consideration for the possibility of side-effects and the possibility that standard doses may be excessive in some circumstances. As a good principle, drugs should only be used when specifically indicated and should be withdrawn as soon as they no longer have a genuine benefit. This principle applies as much to corticosteroids as to any other type of drug.
Complete failure of corticosteroid therapy should be followed by a careful reassessment of the case and, if the diagnosis is confirmed, treatment with aurothioglucose can be attempted as an alternative approach with the same objective. Azathioprine, a potentially toxic immunosuppressive drug, can also be used when corticosteroids are contraindicated for other reasons or fail to bring a resolution.
Systemic corticosteroid therapy (Stannard 1994)
Clinical effects
Drug type
1. Short-acting (biological effects last for less than 12 hours), e.g. hydrocortisone, cortisone
2. Medium-acting (biological effects last for 12–24 hours), e.g. prednisolone or prednisone, triamcinolone, methylprednisolone and isoflupredone
3. Long-acting (biological effects last for over 48 hours), e.g. dexamethasone, betamethasone, flumethasone
4. Slow-release, e.g. methylprednisolone acetate
Route of administration
The preferred route of administration for each of the compounds varies.