The use of intraarticular corticosteroids for treatment of equine OA was extensively reviewed in 1996, and the benefits and deleterious side effects were more recently clarified.17 Based on my observation of an apparent lack of correlation between the previous use of betamethasone esters (Betavet Soluspan, Schering-Plough Animal Health Corp., Union, New Jersey, United States) and articular cartilage degradation during arthroscopic surgery for osteochondral chip removal, experimental studies were initiated for the three most commonly used intraarticular corticosteroids. Methylprednisolone acetate (MPA) (Depo-Medrol, Pharmacia and Upjohn Co., Kalamazoo, Michigan, United States), triamcinolone acetonide (TA) (Vetalog, Bristol Myers Squibb for Fort Dodge, Fort Dodge, Iowa, United States), and betamethasone esters (Betavet Soluspan) were evaluated using an osteochondral fragment–exercise model.18-20 Betamethasone (Betavet Soluspan, now available as Celestone Soluspan) was tested first. Osteochondral fragments were created arthroscopically on the distal dorsal aspect of the radial carpal bone in both middle carpal joints in 12 horses, and one joint was treated with 15 mg of betamethasone at 14 and 35 days after surgery.18 The contralateral control middle carpal joint was injected with saline. No deleterious side effects on articular cartilage were demonstrated. Exercise produced no harmful effects in the presence of betamethasone.18
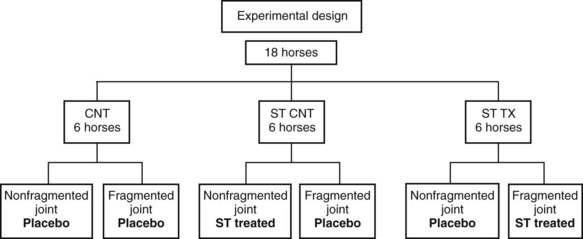
In subsequent studies with intraarticular corticosteroids (as well as other treatments), the research model was modified so that the contralateral joint was not used as a control. The chip fragment model was also modified to more effectively produce early osteoarthritic change. Eighteen horses were randomly assigned to three groups: MPA or TA was injected 14 and 28 days after surgery, and horses were exercised on a high-speed treadmill for 6 weeks, beginning on day 15 after surgery. Results were compared with control joints treated with corticosteroids but which had no osteochondral fragment, as well as with a second control osteochondral fragment group treated with polyionic fluid (Figure 84-1).19,20 In joints containing an osteochondral fragment and treated with MPA, there was a trend (not statistically significant) for lower lameness scores. However, there were significantly lower synovial fluid PGE2 concentrations and lower scores for intimal hyperplasia and vascularity (no effect on cellular infiltration in the synovium compared with placebo-treated joints) in MPA-treated joints compared with control joints. Of more importance, modified Mankin scores (a score of histopathological change in articular cartilage) were significantly increased in MPA-treated joints compared with control joints, suggesting deleterious effects of intraarticular administration of MPA.19 This is in contrast to the results with TA (Vetalog).20 Horses that were given 12 mg of TA in a joint containing a fragment (TA TX) were less lame than horses in the two control groups (see Figure 84-1). Horses treated with TA had lower protein and higher hyaluronan (HA) and GAG concentrations in synovial fluid. Synovium from treated groups had less inflammatory cell infiltration, intimal hyperplasia, and subintimal fibrosis. Analysis of articular cartilage morphological parameters evaluated using a standardized scoring system was significantly better from TA control (no fragment) and TA treatment groups compared with the control placebo-treated fragment group. The results supported favorable effects of TA on lameness scores, synovial fluid, synovium, and articular cartilage morphological parameters, both with direct intraarticular administration and remote site administration compared with placebo injections.20 Evaluation of intraarticular TA on subchondral bone showed no deleterious effects.21 In other work, repetitive intraarticular administration of MPA to exercising horses altered mechanical integrity of articular cartilage but had no effect on subchondral or cancellous bone.23
Based on these and recent in vitro results demonstrating a protective effect of TA,24 I recommend TA be used especially in high motion joints. Some have recommended low doses of MPA to alleviate potential negative effects. However, based on in vitro titration studies, commonly used “low doses” are unlikely to have the same clinical effects because a greater concentration of corticosteroid is needed to inhibit the catabolic compared with the anabolic effects on articular cartilage.25 On the other hand, clinical improvement is seen in horses administered low doses, an observation that is more important to a clinician than are experimental data.
Despite scientific studies demonstrating the efficacy and chondroprotective properties of TA, some practitioners fear the potential of TA to cause laminitis. Laminitis did not occur in 1200 horses treated with TA when the total dose did not exceed 18 mg.26 From this study, 18 mg was established as a maximum dose. A more recent study reported no association between the occurrence of laminitis and the intraarticular use of TA.27 There was a recent legal case in the United Kingdom in which a horse developed catastrophic laminitis after receiving 8 mg of TA in each tarsus and 20 mg of dexamethasone into its back.28 This led to a review of the literature, which revealed that there was a lack of good evidence linking laminitis to corticosteroid injection; it was suggested that a large-scale multicenter trial was needed.29 A related retrospective study from one clinician29,30 revealed that laminitis associated with intraarticular injection of corticosteroids had occurred in 3 of 2000 (0.15%) horses. TA was used the majority of the time, and the upper total dose ranged from 20 to 45 mg.30 The relationship between corticosteroid use and laminitis is discussed further in Chapter 34.
Another traditional cliché is that although it is better not to use MPA in high-motion joints, using it in low-motion joints (such as the distal tarsal joints) is appropriate. This implies that we do not care about the state of the articular cartilage in these joints, and perhaps corticosteroids may promote ankylosis. There is currently no evidence that ankylosis can be promoted in this fashion. The other side of this argument is that we should preserve articular cartilage whenever we can. Intraarticular injection of MPA or TA (with or without hyaluronan [HA]) in horses with OA of the distal hock joints led to a positive outcome in only 38% of horses (suggesting to the authors that surgical treatment may lead to better long-term prognosis).31 There was no significant difference between treatment with either MPA or TA, thus questioning any clinical advantages of the use of MPA.31 However, this was a relatively small study performed on a referral population of horses, and these results may not be representative of the overall response of horses with distal hock OA to intraarticular medication.
Intraarticular corticosteroids are commonly combined with HA, and there is a perception that the HA might be protective against any deleterious effects of corticosteroids (MPA). This perception is based on tradition rather than scientific proof but has become common thinking among equine practitioners.3 Some support can be gained from a 1-year, single-blind, randomized study in which 24 human patients were treated with intraarticular HA once weekly for 3 weeks and then again at 6 months (total of six injections).32 Sixteen of these patients also had TA before the first and fourth HA injections, and using the Western Ontario and McMaster Universities Index of OA (WOMAC) scores, the results were better with the combination of these two products. There was no progression of OA as evaluated using magnetic resonance imaging in either group.32 Two in vitro equine studies evaluated whether HA might have a mitigating effect against the deleterious effects of MPA. In the first study, HA addition had little effect on MPA-induced cartilage matrix proteoglycan catabolism in cartilage explants.33 In the second study, MPA combined with HA had beneficial effects on proteoglycan metabolism in interleukin-1 (IL-1)–treated equine chondrocytes (but there were no comparisons between HA alone and MPA plus HA).34 The combination of MPA and HA increased PG synthesis compared with IL-1–treated controls.34