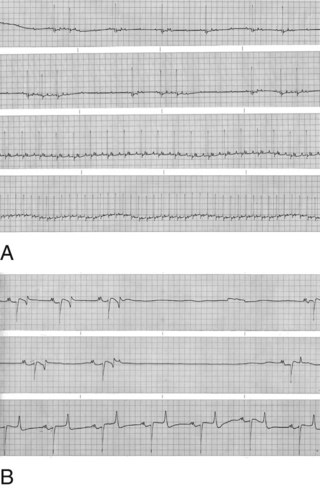
3 A Facilitate physical restraint by modifying behavior (Fig. 3-1) 1. Animal does not resist physical manipulation 2. Animal is not interested in or is oblivious to surroundings B Reduce anxiety, fear, and stress D Reduce or prevent pain before, during, and after surgery E Decrease the dose of drug required to produce sedation, muscle relaxation, analgesia, or general anesthesia F Facilitate safe and uncomplicated induction, maintenance, and recovery G Minimize or prevent the adverse and potentially toxic effects of concurrently administered drugs H Modulate autonomic reflex activity, whether of sympathetic or parasympathetic origin I Anticholinergics (e.g., atropine, glycopyrrolate) A Competitively antagonize acetylcholine at sites innervated by postganglionic, parasympathetic (cholinergic) nerve fibers and on smooth muscles that are influenced by acetylcholine but lack innervation; referred to as parasympatholytics, anticholinergics, or antispasmodics B Primarily used to limit salivary secretions, prevent bradycardia, or deliberately increase heart rate C Inhibit bradycardia caused by reflex increases in vagal tone (e.g., laryngeal or ocular stimulation and vasovagal reflexes) 1. Increasing heart rate generally increases arterial blood pressure (BP) and cardiac output 2. Parasympatholytics may induce a sinus tachycardia or occasionally precipitate ventricular arrhythmias. Anticholinergic drugs may cause sinus bradycardia then progress through first- and second-degree atrioventricular block before the establishment of a faster sinus rhythm (Fig. 3-2). 3. Atropine sulfate may stimulate vagal nuclei in the medulla and, thus, induce an initial sinus bradycardia; not seen with glycopyrrolate 4. Vagal reflexes (bradycardia) produced by traction on visceral organs or during ocular surgery can be but are not always successfully treated with parasympatholytic drugs 5. General anesthetics, opioids, α2-agonists, digitalis glycosides, hyperkalemia, acidosis, and injection of calcium salts augment vagal effects and may precipitate bradycardia a. Opioids and α2-agonists increase parasympathetic tone and can produce sinus bradycardia and first- and second-degree atrioventricular block b. Isoflurane, sevoflurane, and barbiturates indirectly enhance parasympathetic effects by suppressing sympathetic tone c. Phenothiazine tranquilizers rarely produce a CNS-induced cholinergic effect and pronounced sinus bradycardia D Gastric pH is increased (i.e., less acidic); gastrointestinal (GI) motility and contractions of the bladder and ureters are reduced; intestinal motility can be decreased for several hours in horses, an effect that could cause colic. Ruminal atony (bloat) can occur in ruminants E Reduce glandular secretions of the respiratory tract, GI tract, oral and nasal cavities 1. The accumulation of excessive secretions in the oral cavity of small animals (e.g., cats) may predispose to upper airway obstruction or trigger laryngospasm 2. Increased secretory activity may occur after parasympatholytic drug effects subside; this is known as a postparasympatholytic rebound phenomenon F Produce bronchodilation (increased physiologic dead space) and mydriasis G Atropine and scopolamine may produce drowsiness and potentiate the effects of CNS depressant drugs; large doses may stimulate cerebral areas, leading to restlessness, disorientation, and delirium, an effect more common in ruminants and elephants H Glycopyrrolate, a quaternary ammonium drug, does not cross the blood-brain or placental barriers I Administer intramuscularly (IM) or subcutaneously (SQ) (IV for emergencies) 1. Atropine (20 to 40 µg/kg) or glycopyrrolate (10 µg/kg) increases heart rate and dries secretions in small animals. The duration of action of atropine is 60 to 90 minutes; the duration of action of glycopyrrolate is 2 to 4 hours. 2. Parasympatholytics are of questionable value in horses and ruminants because of side effects (bloat, colic) or lack of effect, respectively a. Horses: atropine, 20 to 40 µg/kg; glycopyrrolate, 3.3 to 6.6 µg/kg IV in response to bradycardia b. Ruminants: not recommended for decreasing salivation; atropine temporarily decreases secretions, which can become more viscous; proper positioning of the head and neck is of the utmost importance to prevent pooling of saliva in the pharynx and subsequent aspiration c. Pigs: atropine (20 to 40 µg/kg); glycopyrrolate (3.3 µg/kg) 1. Atropine may slow heart rate transiently after IV administration 2. Cardiac arrhythmias: first- and second-degree atrioventricular block progressing to sinus tachycardia can be observed after IV administration of atropine or glycopyrrolate in animals with sinus bradycardia; ventricular arrhythmias may occur after IV administration 3. Sinus tachycardia increases myocardial oxygen consumption and can precipitate heart failure or pulmonary edema in animals with preexisting cardiovascular disease (heart failure) if heart rate becomes excessively rapid 4. Atropine may cause CNS depression in dogs and cats; it may cause restlessness, delirium, and disorientation in ruminants and elephants 5. Slows intestinal transit time and is thought to cause ileus and colic in horses II Tranquilizers and sedatives (e.g., phenothiazines, butyrophenones, benzodiazepines, α2-agonists; Table 3-1) TABLE 3-1 Intravenous Doses of Commonly Used Tranquilizers and Sedatives Doses are given as mg/kg. *The maximum total dose of acepromazine as preanesthetic medication in dogs is 4 mg. A Phenothiazines (e.g., acepromazine, promazine), butyrophenones (e.g., droperidol) a. Calming and neurologic effects are mediated by depression of the reticular activating system and antidopaminergic actions in the CNS b. Suppress the sympathetic nervous system (depress mobilization of catecholamines centrally and peripherally) c. Phenothiazine tranquilizers may lower seizure threshold in animals with epilepsy, however, they may inhibit chemically induced seizures (e.g., ketamine) d. Phenothiazines and butyrophenones produce antiemetic effects by inhibiting dopamine interaction in the chemoreceptor trigger zone in the medulla b. Can be mixed with other water-soluble drugs (e.g., acepromazine and hydromorphone; acepromazine and ketamine) 3. Produce mental calming, decrease motor activity, and increase threshold for responding to external stimuli a. Not considered analgesic, but improve the analgesic effects of other drugs b. Excessive doses of phenothiazines and butyrophenones can cause involuntary (extrapyramidal) musculoskeletal effects and hallucinatory activity in some animals, particularly horses c. The calming effect can be temporarily reversed with an adequate stimulus; larger doses may be required in excitable or apprehensive animals and may produce disorientation, ataxia, and attempts to escape a. α-Adrenergic blockade results in vasodilation and a decrease in arterial BP (hypotension). Subsequent epinephrine administration may cause a paradoxical drop (β2 effect) in arterial BP because α receptors are blocked. (1) Hypotension occurs more frequently in excited or apprehensive animals. Reflex tachycardia may occur in response to hypotension. Treat with IV fluids or α-adrenergic agonist (phenylephrine: see below). (2) Severe reactions include hypotension resulting in fainting and (rarely) bradycardia (reported in Boxer dogs) resulting in death (3) Phenylephrine (α1-agonist) and IV fluids can be used to increase BP if hypotension is severe b. Heart rate usually decreases as the animal becomes calm; however, reflex tachycardia may occur if the animal becomes hypotensive c. Antiarrhythmic effects: phenothiazines block α1-receptors, produce quinidine-like effects, and decrease central sympathetic, ganglionic, and peripheral (adrenal) activity, reducing the incidence of ventricular arrhythmias d. Dose-dependent depression of the myocardium and vascular smooth muscle 5. Reduce respiratory rate; may decrease tidal volume when administered in large doses; decreases respiratory center sensitivity to increases in carbon dioxide (CO2) 6. Potentiate the ventilatory and cardiovascular depressant effects of α2-agonists, opioids, and drugs used to produce general anesthesia 7. Useful as antiemetics. Acepromazine and droperidol administered 15 minutes before opioids lower the incidence of vomiting. 8. Most have antihistaminic properties; phenothiazines and butyrophenones should be avoided when skin testing for allergies 9. Most phenothiazine tranquilizers cross the placental barrier relatively slowly 10. Many phenothiazine tranquilizers, including acepromazine and promazine, may cause erection (priapism) and temporary or permanent prolapse of the penis in stallions or geldings. Potentially reversible by administering benztropine (20 µg/kg IV). 11. Butyrophenone tranquilizers produce calming and antiemetic effects (see above) 12. Primary organ of metabolism is the liver; should be avoided in animals with moderate to severe liver disease or portocaval shunts 13. Clinical effects are present for 4 to 8 hours but residual effects may last up to 48 hours or longer in older animals or animals with liver disease 14. Acepromazine is the most popular and safe phenothiazine used in veterinary practice; butyrophenone tranquilizers are rarely used in veterinary medicine (Tables 3-1 and 3-2) TABLE 3-2 Intravenous Doses of Commonly Used Opioids and Neuroleptanalgesics Doses are given as mg/kg unless otherwise stated. IM dose is one to two times the IV dose. Lower drug doses should be used in sick animals. a. Tachycardia or (rarely) bradycardia b. Hypotension; decreases in BP may result in transient decreases in packed cell volume and plasma total protein c. Hypothermia secondary to peripheral vasodilation d. Acepromazine significantly decreases platelet numbers and their ability to aggregate; however, this does not produce hemostasis problems in otherwise normal animals. Avoid in animals with von Willebrand’s disease or other clotting abnormalities. e. Akathisia: restless condition in which the patient needs to be in constant motion f. Acute dystonic reactions: hysteria, seizures, ataxia g. Tranquilizers alter behavior, generally producing calming and depression but can increase anxiety and produce restlessness and occasionally aggression in some dogs and cats. Their effects on the reticular formation of the pons and medulla (extrapyramidal effects) can lead to akinesia (inability to initiate movement) and akathisia (inability to remain motionless) particularly in aged animals. B Benzodiazepines (e.g., diazepam, midazolam, zolazepam) are centrally acting muscle relaxants (see Table 3-1) a. Exert many of their pharmacologic effects by enhancing the activity of CNS inhibitory neurotransmitters (gamma-aminobutyric acid [GABA], glycine) by binding to the benzodiazepine site of the GABAA receptor and opening chloride channels, thereby hyperpolarizing membranes. This effect is produced by combining with CNS benzodiazepine (BZ1, BZ2) receptors. Effects can be antagonized by the benzodiazepine antagonist flumazenil. b. Depress the limbic system, thalamus, and hypothalamus (reducing sympathetic output), thereby inducing a mild calming effect and disorientation c. Reduce polysynaptic reflex activity, resulting in muscle relaxation and antiseizure effects d. Stimulate appetite and pica a. Diazepam is formulated in 40% propylene glycol, ethyl alcohol, sodium benzoate, or benzoic acid to solubilize the drug; potentially can produce hypotension, bradycardia, and apnea if administered too rapidly IV b. Midazolam and zolazepam are water soluble c. Diazepam and midazolam are absorbed through the mucosal membranes in the oral cavity and esophagus and in the stomach. Absorption from the solutions or syrups may be significantly improved by slightly increasing the pH (pH 4-6). 3. Recommended doses produce minimal sedation in normal animals; calming effects are observed in sick, depressed, or debilitated animals a. Minimal hypotensive effects are observed after IV administration b. Bradycardia and hypotension may occur after rapid IV administration c. Respiratory rate and tidal volume are minimally affected d. Some antiarrhythmic effects are produced as a result of decreases in sympathetic nervous system activity
Preanesthetic and Perioperative Medications
Overview
Purposes of Preanesthetic and Postanesthetic Drugs
Drug Categories
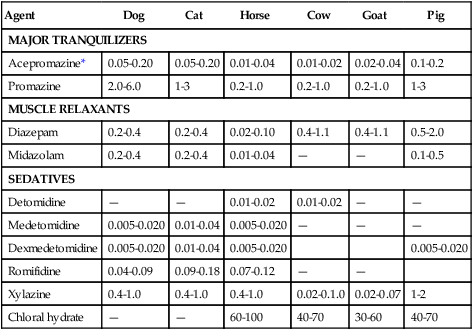
Agent
Dog
Cat
Horse
Cow
Goat
Pig
MAJOR TRANQUILIZERS
Acepromazine*
0.05-0.20
0.05-0.20
0.01-0.04
0.01-0.02
0.02-0.04
0.1-0.2
Promazine
2.0-6.0
1-3
0.2-1.0
0.2-1.0
0.2-1.0
1-3
MUSCLE RELAXANTS
Diazepam
0.2-0.4
0.2-0.4
0.02-0.10
0.4-1.1
0.4-1.1
0.5-2.0
Midazolam
0.2-0.4
0.2-0.4
0.01-0.04
—
—
0.1-0.5
SEDATIVES
Detomidine
—
—
0.01-0.02
0.01-0.02
—
—
Medetomidine
0.005-0.020
0.01-0.04
0.005-0.020
—
—
—
Dexmedetomidine
0.005-0.020
0.01-0.04
0.005-0.020
0.005-0.020
Romifidine
0.04-0.09
0.09-0.18
0.07-0.12
—
—
Xylazine
0.4-1.0
0.4-1.0
0.4-1.0
0.02-0.1.0
0.02-0.07
1-2
Chloral hydrate
—
—
60-100
40-70
30-60
40-70
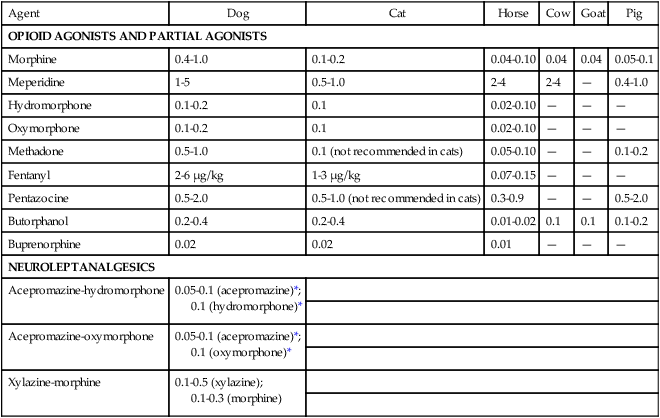
Agent
Dog
Cat
Horse
Cow
Goat
Pig
OPIOID AGONISTS AND PARTIAL AGONISTS
Morphine
0.4-1.0
0.1-0.2
0.04-0.10
0.04
0.04
0.05-0.1
Meperidine
1-5
0.5-1.0
2-4
2-4
—
0.4-1.0
Hydromorphone
0.1-0.2
0.1
0.02-0.10
—
—
—
Oxymorphone
0.1-0.2
0.1
0.02-0.10
—
—
—
Methadone
0.5-1.0
0.1 (not recommended in cats)
0.05-0.10
—
—
0.1-0.2
Fentanyl
2-6 µg/kg
1-3 µg/kg
0.07-0.15
—
—
—
Pentazocine
0.5-2.0
0.5-1.0 (not recommended in cats)
0.3-0.9
—
—
0.5-2.0
Butorphanol
0.2-0.4
0.2-0.4
0.01-0.02
0.1
0.1
0.1-0.2
Buprenorphine
0.02
0.02
0.01
—
—
—
NEUROLEPTANALGESICS
Acepromazine-hydromorphone
0.05-0.1 (acepromazine)*;
0.1 (hydromorphone)*
Acepromazine-oxymorphone
0.05-0.1 (acepromazine)*;
0.1 (oxymorphone)*
Xylazine-morphine
0.1-0.5 (xylazine);
0.1-0.3 (morphine)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



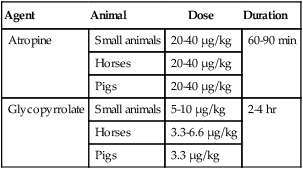
 Anticholinergics
Anticholinergics