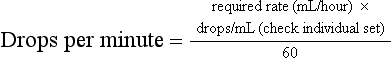Chapter 3 A cornerstone of excellent patient care is careful, appropriate monitoring. Staff should be aware of the importance of the procedures that they perform and the data that they record. For example, essential information will be lost if the litter tray, bedding and cage are cleaned without noting and recording urination. Where urine output is inappropriately low, an opportunity will have been lost for early intervention to prevent acute renal failure (ARF). Minimum monitoring for the postoperative inpatient is a twice-daily examination. A useful approach to direct this examination and to record its findings is the SOAP (subjective, objective, assessment, plan) protocol (Box 3-1). Observing the cat undisturbed in its cage allows assessment of its demeanor and resting respiratory rate and effort, all of which may change upon handling. Unless there are specific indications otherwise, physical examination is best completed on the examination table. Physical examination has been described in Chapter 1. For patients with reduced mobility or where handling causes discomfort, the bedding can be used to support the patient during transfer from the cage (Fig. 3-1). Cats should be weighed using scales that are accurate for small patients, such as pediatric scales (Fig. 3-2). A fall in body weight can accompany dehydration or catabolism, whereas a rapid increase can signal occult effusion. Surgical wounds, drains and feeding tube sites and intravenous (IV) catheter sites should be inspected for discharge, redness, swelling, or evidence of self-trauma. Gentle palpation of surgical sites can assist with pain scoring. The cage should be checked for evidence of urination (estimated volume, color), defecation (amount, color, consistency, presence of mucus, blood), vomitus or hemorrhage. Food and water intake should be recorded. For cats on IV fluids, the rate and type of fluid being administered should be confirmed. Patients receiving IV fluids typically have hematocrit, total plasma protein (TP), electrolytes and, where available, blood gases monitored every 24 hours. Judicious sampling minimizes handling and restraint and avoids exacerbating anemia. Using an insulin syringe for venipuncture is well tolerated, minimally traumatic and provides sufficient sample for microhematocrit, TP, electrolytes, blood glucose and blood film preparation. Subjective and objective data form the basis of the patient assessment. All problems identified should be ranked and assessed (Chapter 1) and an overall assessment of the cat’s status as stable, improving or deteriorating made. Consideration should be given to the pain score, hydration/nutritional status, changes in body weight, new problems, or problems that cannot be accounted for. A pump should be used to deliver IV fluids and a peripheral catheter is suitable for most purposes. Where a fluid pump is not available, a pediatric burette (Fig. 3-3) is preferable to a standard giving set to avoid fluid overload. The required rate is converted to the number of drips/minute for the giving set: Subcutaneous (SC) administration is not suitable for irritant solutions and an unpredictable rate of absorption can precipitate congestive heart failure or hypokalemia. Administration via a feeding tube or the intraosseous route may be indicated. The volume of fluid required for a 24 hour period is the sum of current deficits, maintenance requirements, and ongoing losses (Box 3-2). Losses during surgery include hemorrhage, evaporation from serous surfaces, transudation from traumatized tissues, and gastrointestinal sequestration. The maintenance requirement for an adult cat is approximately 60 mL/kg/day.1 Ongoing losses include those associated with vomiting, diarrhea, polyuria, surgical drains, and transudation into body cavities or externally. Hydration status is estimated based on the patient’s history, physical findings and laboratory data. Changes from objective baseline data such as weight, hematocrit, TP and creatinine are particularly useful. The maximum rate of infusion for cats, 60 mL/kg/hour (90 mL/kg/hour in the dog), is reserved for patients with non-cardiogenic shock, and even then, is given as smaller boluses, repeated as necessary (see below, Management considerations for the septic cat). Care should be taken to avoid interstitial fluid overload, the consequences of which include pulmonary edema, pleural effusion and delayed healing due to prolonged ileus, edema and poor tissue oxygenation. Compensated cardiac and renal disease, chronic anemia, and colloid administration all increase the risk of fluid overload. Urine output should be monitored and maintained above 1–2 mL/kg/hour (or higher where there is obligatory polyuria). Compensated cardiac disease is difficult to detect without echocardiography. Detection of a murmur is neither sensitive nor specific for this purpose.2 Assessment of vertebral heart score, atrial size, and pulmonary vasculature on thoracic radiographs can assist. However, in contrast to dogs, hypertrophic cardiomyopathies are common in cats and significant structural changes can produce minimal change in the cardiac silhouette. Where an increased risk of fluid overload is identified, for example in a cat with advanced hyperthyroidism where significant structural cardiac disease might be predicted despite medical stabilization, conservative fluid rates and additional monitoring should be instituted. Resting respiratory rate and effort can be recorded cage-side, hourly. Increased rate and effort from baseline should prompt assessment for jugular distension or pulsation, thorough auscultation for signs of pulmonary edema or pleural effusion and, if necessary, thoracic radiographs. Serial central venous pressure (CVP) monitoring is useful for avoiding volume overload (normal CVP 0–10 cmH2O).3 As maintenance needs are gradually met from other sources, such as enteral feeding or voluntary food intake, fluid rates should be reduced and then ceased. The exact composition of the fluid administered will depend on individual requirements. In humans and dogs, anesthesia and surgery are associated with a transient increase in antidiuretic hormone (ADH) secretion lasting from one to four days.4 ADH promotes fluid retention and natriuresis. The administration of hypotonic, maintenance solutions in the immediate postsurgical period has been associated with dilutional hyponatremia and fluid overload, which can be severe.5 Assuming that the same is true in cats, then even in a well-hydrated patient, a rehydration solution, such as 0.9% saline or Hartmann solution, is recommended in the initial postsurgical period. Since high rates of potassium-free crystalloids are often administered during surgery, prompt supplementation of fluids after surgery with potassium is necessary to prevent iatrogenic hypokalemia. Hypokalemia results from reduced intake, increased losses or redistribution in alkalosis. In the postsurgical patient, reduced food intake, inadequate supplementation of fluids, solute diuresis, diarrhea, chronic vomiting and diuretic use (loop and thiazide diuretics) can contribute. Other causes of hypokalemia, including primary hyperaldosteronism (Conn syndrome) (Chapter 35), a condition likely underdiagnosed in the cat,6 and breed-associated hypokalemia of Burmese cats, will be identified preoperatively. Intracellular potassium concentrations are actively maintained at much higher levels than extracellular levels and this potassium gradient contributes to the resting membrane potential of excitable cells. When IV fluids are delivered at maintenance rates, supplementation with potassium chloride at 20 mmol/L usually maintains normokalemia. The clinical consequences of severe hypokalemia (<3 mmol/L) include generalized weakness, cervical ventroflexion and reduced gastrointestinal motility. Where the potassium deficit is mild, oral or enteral supplementation can be used (4–10 mmol of potassium gluconate/cat/day divided) or the concentration of potassium in the fluids can be increased up to 40 mmol/L. Where potassium is added to a bag of fluids in use, the fluids must be turned ‘off’ to the patient while adding concentrated potassium solution to prevent infusion until the solution is adequately mixed. The maximum rate of potassium supplementation, 0.5 mmol/kg/hour, should not be exceeded or life-threatening cardiotoxicity can result. If plasma potassium is <3.0 mmol/L, a maximum-rate potassium infusion can be delivered using a syringe driver (Fig. 3-4), or as a secondary infusion on a pump, for one hour, after which electrolytes should be retested and the infusion repeated if necessary (Box 3-3). Hyperkalemia can occur with reduced renal excretion, translocation in acidosis, over-supplementation of fluids and massive tissue necrosis. In the postsurgical period, hyperkalemia should prompt immediate assessment for ARF. Adequate urine output (1–2 mL/kg/hour) should be confirmed without delay and renal parameters determined (plasma creatinine, urea and phosphate). If azotemia is confirmed the contribution of prerenal, renal and postrenal causes should be investigated. Where loss of integrity of the urinary tract is a possibility, e.g., after urinary tract surgery, abdominal ultrasonography is indicated. If an abdominal effusion is identified, simultaneous comparison of creatinine concentration in the plasma and the effusion confirms uroperitoneum, if the creatinine level is higher in the effusion (urea equilibrates quickly and is less useful for diagnosing uroperitoneum). Hyperkalemia in the face of normal renal function can accompany excessive supplementation, acidosis and drug administration (e.g., spironolactone, angiotensin-converting enzyme inhibitors). The most significant clinical consequence of hyperkalemia is cardiotoxicity, manifesting as bradycardia or arrhythmias, and specific treatment with calcium gluconate may be indicated (see Chapter 1 for consequences and treatment of hyperkalemia).
Postoperative monitoring and management of complications
Daily inpatient monitoring
Using the SOAP protocol
Data collection
Assessment
Fluid therapy
Route of fluid administration
Volume of fluid administration
Rate of fluid administration
Fluid composition
Electrolyte abnormalities
Hypokalemia
Hyperkalemia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine