Chapter 7 Postoperative Management
Little has been written about proper postoperative nutritional management of the ruminant patient, even though all successful veterinary surgeons recognize its importance. In the absence of data from controlled clinical trials, this chapter attempts to record contemporary recommendations for postoperative feeding and nutritional support and is based largely upon the author’s opinions and experiences. Nevertheless, the recommendations made consider the physiology and pathophysiology of the relevant animal species and their clinical syndromes. The focus is on strategies for returning the gastrointestinal tract to normal function in the immediate postoperative period with less attention on specific long-term nutritional needs of the patient. More attention must be given to the nutritional needs of neonates and debilitated patients immediately after surgery, including partial and total parenteral nutrition. The nutritional support of these critical care patients is beyond the scope of this chapter.
Evaluation of Gastrointestinal Function
GASTROINTESTINAL FILL
Dorsal and Ventral Distension on the Left with Ventral Distension on the Right
Dorsal and ventral distension on the left with ventral distension on the right indicates rumenoreticular distension—with or without abomasal distension—that is often, but not exclusively, caused by vagal indigestion. Rectal palpation of a full rumen confirms the assessment. Deep palpation of the right ventral flank is recommended to determine whether the abomasum is involved, but experience indicates a distended ventral sac of the rumen can also be palpated in that area. Sonographic examination and visualization of an enlarged fluid-filled abomasum confirms abomasal distension. Evaluating plasma electrolyte values is often necessary to determine whether the abomasum is functioning properly. Hypochloridemia and alkalosis indicate abomasal outflow failure, but postoperative intravenous fluid therapy may obfuscate interpretation of this important preoperative test in the postoperative period.
GASTROINTESTINAL MOTILITY
Evaluation of gastrointestinal motility involves auscultation and palpation. Intestinal and abomasal sounds can be ausculted on the right side and ventrum, respectively. Rumenoreticular motility can be assessed by auscultation or by placing a fist in the left paralumbar fossa to feel the contractions. When evaluating ruminal contractions, one should note both the frequency and strength of contractions. The author prefers palpation to auscultation for initial assessment of ruminal motility. The normal rumen contracts about 2 to 3 times every 2 minutes. More complete evaluation of the rumen can be accomplished by combining auscultation and palpation through the left paralumbar fossa as well as rectally. In addition to frequency and strength of contractions, the physical character of the ruminal contents can be appreciated. A sutured surgical incision in the left paralumbar fossa, often present in postsurgical patients, may complicate the execution of auscultation and palpation in this area. The rumen has two contractile cycles that are called primary and secondary. The primary cycle is associated with mixing ingesta, while the secondary is associated with eructation. The primary cycle stratifies ingesta so the firm fibrous material floats in a mat on top of ruminal liquid. Small particles exit the rumen while larger ones are retained. Plant fibers more than 0.5 cm long in the feces indicate abnormal ruminal contractile activity. The primary cycle is under vagal parasympathetic control. Factors that stimulate contractions include feeding, low environmental temperature, and a slight distension sufficient to stimulate low threshold receptors in the rumen. The low threshold receptors can sometimes be exploited by pumping water and gruel into an empty rumen until mild distension is achieved. This helps stimulate ruminal contractions in anorectic ruminants. Factors that depress ruminal contractile activity include depression, fever, pain, endotoxin, volatile fatty acids, and abdominal distension sufficient to stimulate high threshold receptors. Ruminal motility can sometimes be improved by physically or pharmacologically reversing one or more of these inhibitory factors. Because these stimuli and suppressors of ruminal activity are mediated through the vagus nerve, an intact vagus is required for them to have an effect. Hypocalcemia reduces ruminal contractility by reducing the contractility of smooth muscle fibers irrespective of neural input.
RUMINAL MICROBES
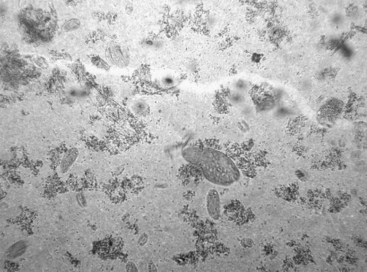
The ruminant forestomachs are a physiologic and biologic wonder, a marvelous example of symbiosis. In the mature cow, the rumen and reticulum represent 64% of the total stomach capacity, whereas the abomasum makes up only 11%. During the transition from preruminant to ruminant, the stomach changes in form, function, and fauna. The rumen and reticulum become a fermentation vat containing between 105 and 1012 bacteria/ml. The vast majority of ruminant microbes of animals on a forage diet are gram-negative anaerobic bacteria. The proportion of gram-positive organisms increases as the amount of grain in the diet increases. The ruminant’s ability to use poor quality roughage, inadequate to sustain nonruminant animals, is facilitated by the bacteria in the rumen. Although bacteria are more important for digestive function, the ruminal protozoa are easier to assess diagnostically, and they provide a reasonable index of ruminal health. Therefore a substantial proportion of the ruminal microbe examination focuses on the protozoal population. For clinical purposes, the ciliate protozoa can be divided into 2 morphologic types: holotrichs and entodiniomorphs. Holotrichs have cilia surrounding their one-celled bodies, whereas entodiniomorphs have cilia at one end (Figure 7.1-1).
Ruminal Fluid Analysis
Indications for clinical evaluation of ruminal microflora include suspicion of ruminal acidosis (e.g., carbohydrate engorgement), vagal indigestion, abomasal emptying defect of sheep, and rumen atony. Sometimes the analysis precedes surgery, but correction of the problem occurs during the postoperative period. A weighted stomach tube or a needle and syringe can be used to collect ruminal fluid for analysis. When a weighted collection tube is used, it is simply passed into the rumen, pushed back and forth to sink the tube, and aspirated. The first 100 ml or so is discarded to reduce salivary contamination. Aspirating transabdominally through the left flank by using a 16- to 18-gauge, 5-inch needle also helps eliminate salivary contamination.
Direct microscopic examination of fresh ruminal fluid on a slide is a quick and useful way to assess the health of the ruminal microflora. Abundant, live, active protozoa of various sizes and shapes will be present in cattle with a normal rumen (see Figure 7.1-1). Very large entodiniomorphs are the most fragile species; their presence suggests a healthy rumen. For further evaluation of the microflora, a drop of Lugol’s iodine can be added to a few drops of fresh rumen fluid. Lugol’s iodine kills the protozoa and stains carbohydrate in protozoa and bacteria. If the protozoa are depleted of carbohydrate, this indicates a depletion of carbohydrate in the rumen. Transfaunation of such an animal without concomitant force-feeding is likely to be ineffective because the newly introduced fauna will not have the substrate to allow them to multiply. Gram staining of the ruminal bacterial population, except in carbohydrate engorgement, has not been diagnostically useful in the author’s experience. If gram staining is performed on ruminal contents, one should expect to see primarily gram-negative organisms of a size and shape quite different from those encountered elsewhere in veterinary medicine. In CHO engorgement, chains of the gram-positive cocci Strep bovis proliferate first; then the large gram-positive rods of Lactobacillus sp become the predominant bacterial type.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree