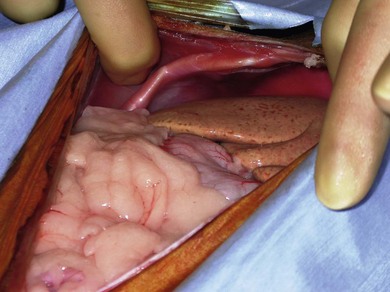
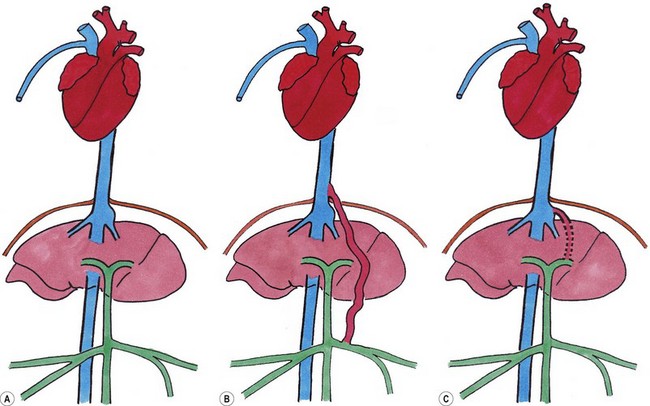
Chapter 32 ‘Portosystemic shunting’ is a broad term that has several possible underlying causes. In cats the main cause of portosystemic shunting is a congenital portosystemic shunt (CPSS), although even this is seen uncommonly, with a reported incidence of 2.5 per 10 000 cats treated in referral practice.1 This chapter provides a comprehensive summary of feline shunts, their diagnosis and surgical management. Normal feline portal vein and caudal vena cava anatomy is depicted in Fig. 32-1. CPSS can take the form of an extrahepatic (outside the hepatic parenchyma) or an intrahepatic (lying partly or wholly within the hepatic parenchyma) vessel. Extrahepatic CPSSs are the most common form, accounting for 73–100% of CPSSs in cats.1–11 The most commonly reported extrahepatic CPSS in cats is an abnormal vessel connecting the left gastric vein to the caudal vena cava6,9,12,13 (Fig. 32-1B). Other extrahepatic shunt morphologies described in cats include porto-azygous, portocaval and shunting vessels between the colonic vein or gastrosplenic vein and caudal vena cava.7,8,10,12,14,15 The majority of intrahepatic CPSSs in cats are due to a patent ductus venosus (PDV), which results from failure of this normal embryonic vessel, on the left side of the liver, to close after birth16,17 (Fig. 32-1C). Right-sided and centrally located intrahepatic shunts have also been reported in cats. Figure 32-1 Feline portal vein anatomy and congenital portosystemic shunts. (A) Normal feline portal vein and caudal vena cava anatomy. (B) The most common extrahepatic CPSS in cats is an abnormal vessel between the left gastric vein and the caudal vena cava. (C) The most common intrahepatic CPSS in cats is a PDV, which empties into a venous dilatation (ampulla) just prior to entering the caudal vena cava in a post-hepatic location in front of the diaphragm. Cats with a CPSS are typically young (under six months of age) but the condition is also recognized in mature animals which may not have exhibited clinical signs for months or years.1,6,7,10,12,14,15,17–20 Although the majority of affected cats are domestic short-haired breeds, a number of pedigree breeds have been suggested as being predisposed, including the Persian, Himalayan, and Siamese.1,6,8,10–12,17,20,21 The clinical signs associated with a CPSS can be variable and non-specific. Signs often wax and wane and can be intermittent. Some cats can essentially appear ‘normal’ and the condition may only be identified as a result of abnormalities seen on routine blood tests in older cats. However, the vast majority of cats (93–100%) present with some degree of neurologic abnormality due to hepatic encephalopathy: including seizures, ataxia, central blindness, behavioral changes, aggression, lethargy, head pressing, and tremors.5–7,12,20 These neurological signs are typically intermittent and can be triggered by or worsen following feeding, gastrointestinal bleeding, or azotemia.1,5,18 Another hallmark of CPSS is ptyalism (hypersalivation), which is reported in 67–84% of cats.5,7,10,12 Cats may be small or stunted with a low body condition score, have copper-colored irises (13–64%) (Fig. 32-2), or intolerance to anesthetics or sedatives.1,6,7,10,12,13,18,20 Other clinical signs include gastrointestinal signs (vomiting and diarrhea), urinary tract signs (dysuria, hematuria or stranguria relating to urate urolithaisis), and polyuria/polydipsia, but these signs are not as commonly seen in cats as in dogs.1,6,7,12 Biochemical and hematological changes are typically mild and non-specific and may be largely unremarkable in cats with CPSS. Performing routine hematological and biochemical analysis of blood may be most useful to rule out other differential diagnoses in the investigation of cats with suspected CPSS. The most common abnormality is low urea due to reduced synthesis of urea in the liver as the product of ammonia metabolism (Krebs–Henseleit urea cycle).10,12,13,22 Hypoalbuminemia, hypoproteinemia, hypoglycemia and anemia are less frequently reported compared with dogs with CPSS.1,10,12,13 There have not been any studies examining clotting abnormalities in cats with CPSS but these have been reported in affected dogs.23,24 The presence of ammonium biurate crystals in urine sediment increases the suspicion of CPSS. A bile acid stimulation test is the most accurate test available to measure liver function for the diagnosis of hepatobiliary disease. It is very reliable for the diagnosis of portosystemic shunting in cats, with a sensitivity of 58–100% (preprandial) and 100% (postprandial), and a specificity of 84% (fasting).1,10,12,25,26 Fasting ammonia is increased in the majority of cats with CPSS but can occasionally be normal.6,10,12,14,18,22,25 The sensitivity and specificity of ammonia for the diagnosis of CPSS in cats has been reported as 83% and 86%, respectively.25 Ammonia is very labile so samples must be analyzed very quickly e.g., using a ‘point of care’ ammonia analyzer, which has been shown to have potential in cats.27 The most likely diagnosis for portosystemic shunting is a CPSS; other very rare differentials include multiple acquired portosystemic shunts (secondary to severe liver disease and portal hypertension), primary portal vein hypoplasia, and arteriovenous fistula.2,3,4 Management of multiple acquired shunts involves treatment of the underlying liver disease if possible and medical management of HE (Table 32-1). Primary portal vein hypoplasia was previously called microvascular dysplasia and is described as microscopic portosystemic shunting within the liver which results in similar clinical signs to animals with a gross CPSS and is managed using medical therapy for HE (Table 32-1). In some animals, primary portal vein hypoplasia is associated with hepatic fibrosis and portal hypertension and this can also lead to the development of multiple acquired shunts. Primary portal vein hypoplasia is a specific disease that should not be confused with underdevelopment (unfortunately also referred to as hypoplasia) of the portal vein. Cats with an extrahepatic CPSS often have an underdeveloped/small portal vein secondary to diversion of blood flow away from the portal vein into the shunt. Hepatic arteriovenous fistula is a communication between the arterial and venous systems e.g., hepatic artery to portal vein, and treatment is by removal of the liver lobe(s) containing the abnormal vascular structures. Plain abdominal radiographs may be performed as part of a diagnostic workup but cannot be used alone to diagnose a CPSS. Abdominal ultrasonography can be extremely useful, particularly as it is relatively inexpensive and non-invasive, but unfortunately it is highly operator dependent. Ultrasonography (Fig. 32-3) has been reported to have a sensitivity and specificity of 100% for shunt diagnosis and type in one study,20 whereas other studies have only identified approximately 47–50% of CPSS in cats.1,10 Per rectum portal scintigraphy with technetium pertechnetate (99TcmO4−) can be very reliable in the diagnosis of CPSS in cats, with good sensitivity and specificity1,12,28,29 (see Chapter 8). However, this is not readily available and its use may be limited in some countries by radiation safety guidelines and availability. Scintigraphy does not provide anatomical information regarding the shunt for preoperative surgical planning, although it may have a role in the follow up of surgically treated cats to assess for continued shunting.29 Figure 32-3 Abdominal ultrasound image of a cat with an extrahepatic CPSS. St, stomach; pv, portal vein; *, shunt branching from portal vein and bypassing the liver. (Courtesy of Chris Lamb.) Many different techniques involving contrast imaging have been described, including cranial mesenteric angiography, splenoportography, and mesenteric portovenography.21,30,31 For intraoperative mesenteric portovenography, contrast is injected through a catheter into a jejunal vein and imaged using a mobile fluoroscopy unit or a mobile radiography unit (Fig. 32-4). Intraoperative portography allows the imaging and surgical attenuation of the shunt to be performed concurrently and avoids the necessity to move the cat into radiography for the imaging or the requirement for two procedures.17 While intraoperative mesenteric portovenography is not essential for every case, it is a quick procedure (approximately ten minutes) and easy to perform with the right equipment. Figure 32-4 (A) The ‘C-arm’ of a mobile digital fluoroscopy unit has been positioned around the operating table over the region of the shunt. (B) Intraoperative portovenograms are then obtained by injecting contrast through a catheter into a jejunal vein. Confirmation of the presence of a shunt and its morphology using portovenography is particularly useful if a CPSS could not be identified preoperatively and/or if the shunt is not easily identified at surgery (more likely if the shunt is intrahepatic). Additionally, portovenography rules out the presence of a second congenital shunt (very rare) or multiple acquired shunts (these may be mistaken for a CPSS if one is much larger than the others). Repeating portovenography after temporary intraoperative occlusion of the shunt confirms the correct vessel has been identified as the shunt and provides an assessment of the degree of intrahepatic portal vascular branching, which may be used to help decide if a CPSS will tolerate complete permanent attenuation (Fig. 32-5). One study examined the extent of intrahepatic vasculature development as assessed on portovenography in cats with CPSS and found it was useful as a prognostic indicator.6 Figure 32-5 Ventrodorsal portovenograms from a cat with a left gastric extrahepatic CPSS. Contrast has been injected through a jejunal vein under fluoroscopic guidance with digital subtraction. The images are orientated so that cranial is at the top. VC, vena cava; PV, portal vein; S, shunt. (A) The majority of the contrast flows through the large shunt vessel (S). The portal vein (PV) and its intrahepatic branches are faint (less contrast opacifying them compared to the shunt). There is minimal intrahepatic portal vein branching. (B) Repeat portovenogram following temporary intraoperative occlusion of the shunt demonstrates increased contrast opacification of the liver and a vast increase in intrahepatic portal vein branching. There is no contrast progressing beyond the point of temporary shunt attenuation. There have been reports of the use of computed tomography (CT) angiography or magnetic resonance angiography (MRA) for diagnosing CPSS in dogs.32,33 Although only one report of the use of CT angiography in a cat with CPSS has been published it is likely that both techniques will be used increasingly to provide a definitive diagnosis and detailed information regarding shunt morphology and intrahepatic vasculature development prior to surgery.34 Surgery involving some form of shunt attenuation is the recommended treatment for most cats with a CPSS. The purpose of surgery is to identify the shunting vessel and to attenuate it in order to redirect blood flow to the liver and improve hepatic function. Thus surgical treatment should theoretically offer the best long-term outcome for the individual. There have not been any studies comparing surgical and medical treatment in the cat. However, a study comparing surgical and medical treatment of CPSS in dogs concluded that in terms of overall survival time surgical treatment was preferable.35 Many cats with a CPSS show relatively severe clinical signs at the time of diagnosis and medical management is recommended to stabilize their condition prior to definitive surgical treatment. In one study of 25 cats managed medically prior to surgery there was no response to medical treatment in 12%, a partial response in 56%, and 32% of cats had complete resolution of their clinical signs.6 Medical management of cats with CPSS treats the signs of HE but does not correct the underlying portosystemic shunting. Medical therapy (see Table 32-1) aims to reduce ammonia (and other toxin) levels, which usually significantly improves the clinical signs of HE. In some instances cats can be presented collapsed, comatose, or in status epilepticus due to severe HE and in these cats additional investigations and therapies are required (Table 32-2). A minimum database (including blood glucose and serum electrolytes) should be performed to rule out other potential causes of seizure or collapse such as electrolyte abnormalities or hypoglycemia and to identify any complicating factors. Cats should be given intravenous fluid therapy with appropriate supplementation to correct any hypoperfusion, dehydration, or metabolic abnormalities, which may be potentiating the problem. In a comatose or seizuring cat it will not be possible to administer drugs orally and parenteral antibiotics are given, although they are likely to be less efficacious than oral antibiotics. The authors recommend intravenous amoxicillin-clavulanate but neomycin can be given rectally as an alternative. Lactulose can be administered as a retention enema. A warm water cleansing enema is administered prior to medicated enemas. The cleansing enema will remove feces and hence reduce ammonia absorption from the colon. If there is evidence of gastrointestinal hemorrhage then omeprazole (a proton pump inhibitor) is recommended. Cats can suffer seizures for a variety of reasons and will often receive diazepam as a first-line treatment. However, there is some controversy over the use of diazepam in cats with CPSS due to concerns over increased GABA and GABA receptors in cats with HE. Cats that do not respond to diazepam and aggressive treatment of HE should be treated with phenobarbitone intravenously. Once the cat is stabilized oral phenobarbitone can be started. If seizures are not controlled by intravenous phenobarbitone administration then it may be necessary to treat the cat with propofol either by bolus or infusion to control the seizures for 24–48 hours before attempting downstaging back to phenobarbitone alone. Propofol infusions should be used with care in cats as prolonged use is associated with delayed recoveries and may in rare cases cause Heinz body anemia. Cats treated with phenobarbitone and propofol infusions should be closely monitored, with regular measurement of pulse rate, respiratory rate, temperature and blood pressure. Endotracheal intubation may be necessary in some instances. Levetiracetam is a relatively new antiseizure medication that has been used in cats, although there are no published reports of its use in cats with HE.36 In cats with severe HE, cerebral edema can develop and may contribute to neurologic signs. In these situations a single bolus of mannitol can be administered to see if this improves the clinical signs. Mannitol should not be used in severely dehydrated or hypoperfused animals. Monitoring progression and response to therapy of severely encephalopathic cats may be facilitated by use of a modified Glasgow coma scale described in dogs.37 Surgical management of CPSS typically requires advanced surgical and critical care facilities: advice on anesthetizing cats with a CPSS is given in Box 32-1. Careful, meticulous planning is required prior to surgery. Shunt attenuation is generally not performed as an emergency procedure so all the necessary preoperative investigations, blood tests, imaging, and stabilization should have been conducted, and a comprehensive plan made both for surgery and for the postoperative care. Concurrent procedures such as removal of calculi, neutering, and hepatic biopsy may need to be performed. Liver biopsy is recommended at the same time as surgical correction of CPSS in cats. This allows assessment of the underlying pathology and identification of any additional pathology. Grossly, the liver of a cat with a CPSS is usually smaller in size and may have abnormal coloration (Fig. 32-6). Typical histopathologic changes associated with CPSS include lobular atrophy, hepatocyte degeneration, reduced or absent portal veins, arteriole hyperplasia, biliary hyperplasia, dilated lymphatics, and occasionally mild portal fibrosis.7,10,22,29 No studies in cats have investigated the relationship, if any, between the histopathologic changes and outcome, although none has been identified in dogs with CPSS.38,39
Portosystemic shunts
Surgical anatomy
Diagnosis and general considerations
Signalment and clinical signs
Biochemical and hematological investigations
Differential diagnoses
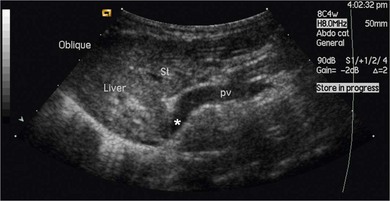
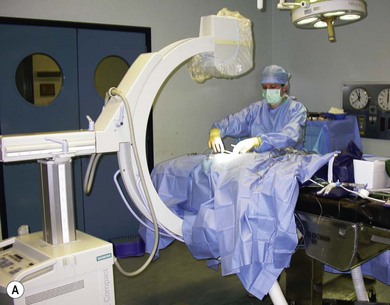
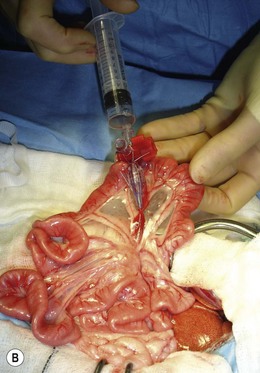
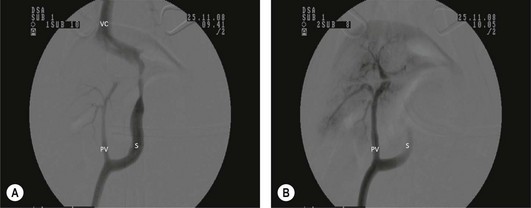
Diagnostic imaging
Preoperative stabilization
Stabilization and medical management
Preoperative seizure management
Preparation for surgery
Concurrent procedures during shunt attenuation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Portosystemic shunts