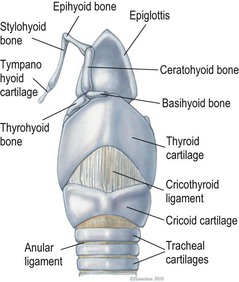
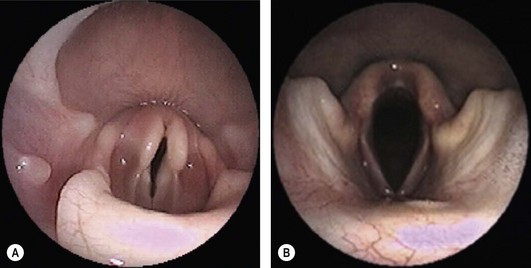
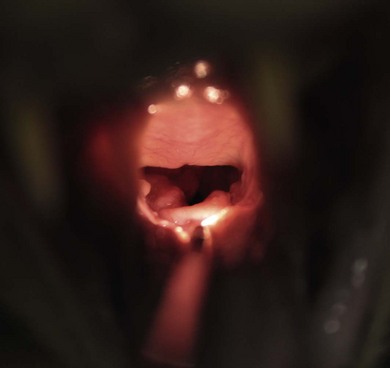
Chapter 52 The larynx is situated between the pharynx and first tracheal cartilage ring. It serves as a conduit for passage of air between the pharynx and trachea. The larynx functions in phonation, regulation of airflow through its lumen, and protection of the lower airway during swallowing. The larynx consists of a set of complicated cartilages, ligaments, and muscles (Figs 52-1 and 52-2). The five laryngeal cartilages are the epiglottis, thyroid cartilage, cricoid cartilage, and paired arytenoid cartilages. The epiglottis is the most rostral of the laryngeal cartilages. It is triangular in shape and composed of flexible fibrocartilage. It is attached by a fold of mucous membrane to the root of the tongue.1 The epiglottis, together with the laryngeal adductors, covers the laryngeal opening during swallowing, allowing food and fluid to pass over it and into the esophagus.1 The thyroid cartilage is the largest of the laryngeal cartilages. It is U-shaped, continuous ventrally and forms approximately two-thirds of the circumference of the larynx. It is attached cranially to the hyoid bone and articulates dorsally with the cricoid cartilage. The cricoid cartilage is signet ring shaped, with its broader part dorsad. Its caudal border is connected to the first tracheal ring. It articulates with the arytenoid cartilages on each side in addition to its thyroid cartilage articulation. The paired arytenoid cartilages are pyramidal in shape, with the base and sides nearly equilateral triangles. The arytenoid cartilages articulate with the cricoid and thyroid cartilages. Figure 52-1 The feline larynx ventral view (arytenoid cartilages not visible) with hyoid apparatus on the right-hand side. (Medical illustrations © Wm. P. Hamilton 2010.) Figure 52-2 Illustration of the intrinsic musculature of the feline larynx, lateral view with window in thyroid cartilage. ATM, arytenoideus transversus muscle; ADM, arytenoideus dorsalis muscle (more commonly referred to as cricoarytenoideus dorsalis muscle); TM, thyroarytenoideus muscle; CLM, cricoarytenoideus lateralis muscle; CM, cricothyroideus muscle; VM, ventricularis muscle. (Medical illustrations © Wm. P. Hamilton 2010.) The vocal folds, thyroarytenoid muscles, and cricoarytenoid dorsalis and lateralis muscles attach to the arytenoid cartilages. Movements produced by these muscles and the vocal folds, open and close the rima glottidis, the laryngeal airway. There are two pairs of tissue folds at the cranial end of the feline larynx; the false vocal cords that lie more cranially and laterally, while the true vocal cords lie more caudally and nearer the median plane. The laryngeal ventricles are reported to lie between the true and false vocal cords on each side.2 However, laryngeal ventricles are also reported not to exist in feline species.3 To the author’s knowledge, eversion of laryngeal ventricles has not been described in the cat, though this is commonly observed in dogs with brachycephalic upper airway syndrome. The false and true vocal cords are most easily visualized by ventral laryngotomy.2 The false vocal cords, often appearing tannish in color, extend from the arytenoid cartilages to the epiglottis. The true vocal cords, appearing as whitish bands, extend from the arytenoid cartilages to the thyroid cartilages. Conflicting reports of the presence of muscle in feline vocal cords exist, with one report stating that feline vocal cords do not contain muscle, in contrast to the dog and horse,4 while another report cites extremely rapid twitching of the vocalis muscle running within the vocal folds as the mechanism of purring.5 The feline cuneiform and corniculate processes are inconspicuous when viewed per os (Fig. 52-3A), while these structures are easily identified in dogs (Fig. 52-3B).2 The larynx is elevated and depressed by action of the thyrohyoideus and sternothyroideus muscles, respectively. The laryngeal lumen is lined by mucous membrane. Figure 52-3 (A) Per os endoscopic view of normal feline larynx. (B) Per os endoscopic view of normal canine larynx. (Photographs courtesy of Dr Nicole van Israël, ACAPULCO cardiopulmonary referrals, Masta, BE.) The larynx is innervated by branches of the vagus. The vagus gives rise to the cranial laryngeal nerve which separates into an internal and external branch.6 The cricothyroid muscle is innervated by the external branch, while the internal branch provides sensory innervation to the laryngeal muscles and mucosal surfaces.6,7 The recurrent laryngeal nerves separate from the vagus at the thoracic inlet. The right recurrent laryngeal nerve passes around the subclavian artery before coursing cranially and traveling up the neck, while the left recurrent laryngeal nerve passes around the aortic arch on the left side before coursing cranially.4 The recurrent laryngeal nerves terminate as caudal laryngeal nerves, innervating ipsilateral thyroarytenoideus, arytenoideus, and cricoarytenoideus muscles. The cricoarytenoideus dorsalis muscle is the only intrinsic laryngeal muscle responsible for abduction of the vocal cords, thus its critical role in respiratory dysfunction in laryngeal paralysis. In all species studied, the left recurrent laryngeal nerve is more commonly involved than the right in laryngeal paralysis. The left recurrent laryngeal nerve has a longer course and fewer nerve fibers compared to the right,4 which is postulated to account for its susceptibility to injury and the more common occurrence of left laryngeal hemiplegia. Interestingly, experimental left recurrent laryngeal denervation by resection of 30 mm length of the nerve in cats did not result in clinical signs of respiratory obstruction up to one month after surgery.8 At one month, functional electrical stimulation of the laryngeal adductors restored mobility of the vocal folds and improved vocalization.8 Clinical signs of laryngeal disease in cats are often vague and non-specific. Most commonly reported clinical signs include tachypnea or dyspnea, inspiratory noise or stridor, dysphagia, weight loss, change in vocalization, coughing independent of eating, coughing and gagging when eating, and lethargy. Absence of purring is reported in cats with laryngeal paralysis and resumption of purring has been reported after surgery in some cats.4 Absence of purring and a change in voice is also reported in cats with laryngeal tumors.9 Laryngeal stridor in cats with laryngeal paralysis has been described as frequently having a distinct whistling component that can be less easy to recognize as part of the respiratory cycle.7 Detection of this whistling sound may be facilitated by auscultation of the laryngeal region. Careful palpation of the cervical area to detect masses and compression of the cranial thorax to assess for a mediastinal mass are also important in the physical examination of cats with suspected laryngeal disease. Radiographic examination of the thorax and neck is recommended, but extreme care in restraint and positioning is required. Sedation or general anesthesia may be required in some cases to obtain diagnostic quality radiographs. Radiographic evidence of airway obstruction may be observed, including hyperinflation of the lungs, prominent caudal displacement of the larynx, and presence of air in the pharynx, larynx, esophagus, and stomach. Mass lesions in the larynx, cervical soft tissues, thoracic inlet, or thorax may be observed (Fig. 52-4). Sonographic examination of the larynx has been reported in 30 cats.10 In five of these 30 cats, the larynx was normal. Vocal fold movement in the normal cats was difficult to identify. The presence and location of cysts and masses were accurately identified in nine cats and the authors considered echolaryngography likely superior to laryngoscopy for identification of cysts and soft tissue masses and cited the clinical advantage that echolaryngography could be performed in these animals without sedation or anesthesia. In eight cats with laryngeal paralysis and/or laryngeal collapse, poor movement of the vocal folds was observed, but further studies and further refinement of the technique are needed for its use in laryngeal paralysis. The use of advanced imaging techniques in the evaluation of laryngeal conditions in the dog has been reported.11 Findings of laryngeal paralysis were identified by CT in unanesthetized dogs, including failure of arytenoid cartilage abduction and a narrow rima glottis. CT and MRI have not (to the author’s knowledge) yet been evaluated in the cat, but based on the canine study these imaging modalities may have a role to play in the evaluation of laryngeal conditions in this species, although the precise nature of their role remains to be fully defined. Definitive diagnosis of feline laryngeal disease is most often achieved by direct laryngoscopy per os under a light plane of anesthesia. Direct laryngoscopy can be achieved with a handheld laryngoscope or an endoscope. Direct laryngoscopy allows assessment of laryngeal function as well as observation for laryngeal masses (Fig. 52-5) or other lesions, which can usually be sampled by fine needle aspiration or biopsy per os. Anesthesia is required for direct laryngoscopy. Protocols for sedation and anesthesia appear to vary widely. Similar to other species, general anesthesia may obliterate laryngeal function in normal animals. Particular care is required to distinguish sedative or anesthetic drug-induced laryngeal paralysis from the clinical disease. Additionally, it is important to recognize the potential presence of paradoxical laryngeal movement which could be mistaken for normal laryngeal abduction/adduction; this can be avoided by assessing laryngeal movement in time with each phase of the respiratory cycle. Care is also required to avoid contact with laryngeal structures during direct laryngoscopy due to the propensity for laryngospasm in cats. Complete recovery of base-line response after spraying the larynx of normal cats required 17–100 minutes for 2% lidocaine and 61–256 minutes for 10% lidocaine in one study.12 Assessment of laryngeal function is therefore impossible following local anesthetic administration. Figure 52-5 Endoscopic view of a feline laryngeal soft tissue mass. Histologic diagnosis was squamous cell carcinoma. (Photograph courtesy of Jon Hall, Cambridge University, UK.) A prospective, randomized clinical trial of anesthetic induction agents for assessment of laryngeal function by videoendoscopy in healthy adult cats premedicated with methadone has recently been reported.13 Induction by slow infusion of midazolam and ketamine to effect preserved arytenoid movement better in comparison to induction with propofol or alfaxolone. However, arytenoid movement was completely obliterated in three cats in the midazolam-ketamine group and three cats in the propofol group. The obliteration of laryngeal function in these normal cats highlights the difficulty of making a definitive diagnosis of laryngeal paralysis in this species. Competent laryngeal protective reflexes were maintained in normal cats anesthetized with ketamine and administered barium suspension orally.14 Barium was aspirated into the pharynx and cranial part of the trachea in these cats, but not into the lower respiratory tract. Administration of doxapram hydrochloride to stimulate more vigorous laryngeal motion in anesthetized patients to assist in the diagnosis of laryngeal paralysis has been described in healthy dogs15 and recommended by one author as a useful technique in the cat.16 Safety and efficacy of doxopram administration in cats with laryngeal paralysis has not been documented, to the author’s knowledge. No breed or sex predilection has been reported yet for feline laryngeal paralysis. A congenital form of laryngeal paralysis has been hypothesized with the observance of laryngeal paralysis in kittens and cats less than two years or age; however, this form of the disease appears to be exceedingly rare.17 In contrast to dogs, where laryngeal paralysis is most often considered to be idiopathic or part of a poorly characterized general neuropathy, laryngeal paralysis in cats is often non-idiopathic in origin. Feline laryngeal paralysis has been diagnosed in association with neoplastic infiltration of the vagus nerve in lymphosarcoma, in extranodal lymphosarcoma, as an iatrogenic complication of thyroidectomy and other surgical procedures of the neck, as a symptom of lead toxicosis, and as part of a progressive generalized neuromuscular disorder.17,18 Unilateral laryngeal paralysis has also been observed in a cat with clinical signs of peripheral vestibular disease, ataxia, and dyspnea diagnosed with adenocarcinoma of the tympanic bulla and Wallerian degeneration of the right recurrent laryngeal nerve.6 So-called idiopathic laryngeal paralysis also occurs in cats. However, the frequency of non-idiopathic etiology of feline laryngeal paralysis warrants careful and thorough clinical and diagnostic evaluation of all cats with suspected laryngeal paralysis for underlying specific etiology. Ninety-nine cats with a diagnosis of laryngeal paralysis were identified in a search of the Veterinary Medicine Data Base from January 1986 through April 2008.19 Fourteen of these 99 cats met the inclusion criteria for their evaluation of unilateral arytenoid lateralization for the treatment of laryngeal paralysis in cats. Proposed etiology of laryngeal paralysis in these animals included congenital (one cat), traumatic (two cats), idiopathic (seven cats), polyneuropathy/polymyopathy (three cats), and iatrogenic (one cat). Concurrent disease, most commonly azotemia, was reported in 11/14 cats. Ten of 14 cats were diagnosed with bilateral laryngeal paralysis, three were left unilateral, and one was right unilateral. All 14 cats were treated with unilateral arytenoid lateralization. Minor variations in technique included degree of disarticulation of the larynx; suture placement (arytenoid to thyroid versus arytenoid to cricoid cartilages); and type, size, and number of strands of suture material used. Intraoperative complications were observed in three of 14 cats (21%) including fracture of the muscular process in two and laryngeal displacement in one. Postoperative complications were reported in seven of 14 cats (50%) but were treatable and/or considered minor. All 14 cats were discharged alive and there was no reported aspiration pneumonia. The incidence of aspiration pneumonia in dogs following unilateral arytenoid lateralization is 8–18%. The authors concluded that the prognosis for resolution of respiratory compromise in cats with laryngeal paralysis treated with unilateral arytenoid lateralization appears good. In a retrospective study of laryngeal paralysis in 16 cats,17 bilateral laryngeal paralysis was reported in 12 cats. In the cats with unilateral laryngeal paralysis, the left side was affected. Three of the four unilaterally affected cats were managed medically, while seven of 12 bilaterally affected cats were managed surgically. Long-term outcome was considered successful in four of seven surgically managed cats. A variety of surgical techniques were used in these cats including unilateral or bilateral arytenoid lateralization, ventriculocordectomy, and partial arytenoidectomy. A retrospective study of ten cats, with arytenoid lateralization for laryngeal paralysis was reported by Hardie et al.20 Nine of these ten cats had bilateral laryngeal paralysis. Concurrent medical problems were diagnosed in seven of these ten cats, including hyperthyroidism, renal insufficiency, congestive heart failure, hypertension, thyroid adenocarcinoma, and mycoplasma pneumonia. Of these ten cats, seven had unilateral arytenoid lateralization, one had bilateral arytenoid lateralization, and two had staged bilateral procedures. All three cats with bilateral procedures developed aspiration pneumonia and died.
Larynx
Surgical anatomy

General considerations
Clinical examination
Diagnostic imaging
Radiography
Sonography
Computed tomography (CT)/magnetic resonance imaging (MRI)
Laryngoscopy
Surgical diseases
Laryngeal paralysis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine