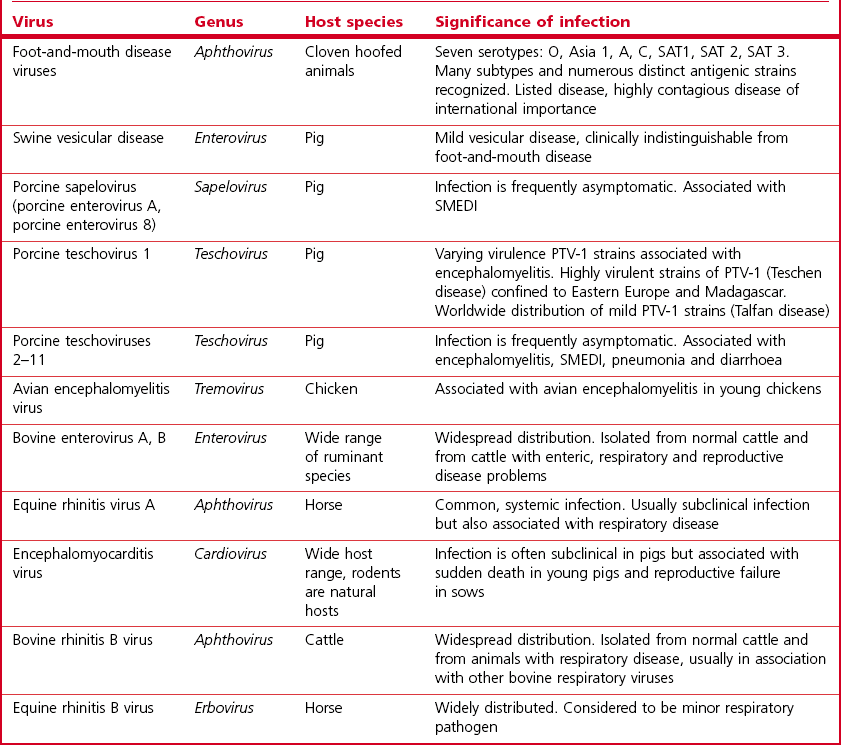
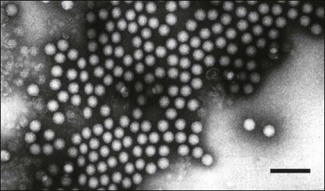
Chapter 52 Picornaviruses get their name from the prefix ‘pico’ meaning very small and the abbreviation for ribonucleic acid. In keeping with their name these viruses are only 22–30 nm in diameter and contain a single molecule of infectious, positive-sense, single-stranded RNA. Individual virions are icosahedral and non-enveloped (Fig. 52.1). The capsid is composed of 60 protein subunits. Each subunit consists of four proteins VP1 (1D), VP2 (1B), VP3 (1C) and VP4 (1A). The protein VP4 is located on the inner surface of the capsid. Viral replication occurs in the cytoplasm of cells in membrane-associated complexes. Infection is usually cytolytic. The family is part of the order Picornavirales and is divided into 12 genera (Fig. 52.2): Enterovirus, Teschovirus, Cardiovirus, Aphthovirus, Hepatovirus, Erbovirus, Kobuvirus, Parechovirus, Tremovirus, Sapelovirus, Avihepatovirus and Senecavirus. The human rhinoviruses, a major cause of the common cold in man, have been moved to the genus Enterovirus and the genus Rhinovirus removed completely. Significant re-classification of the porcine enteroviruses has occurred with many being transferred to the newly created genus Teschovirus. Many former avian enteroviruses have been re-named and re-assigned to another genus or left unassigned. Avian nephritis virus 1, 2 have been re-classified in the family Astroviridae. The genera Hepatovirus and Parechovirus contain viruses of human importance including hepatitis A virus. Seneca Valley virus has a marked affinity for human tumour cell lines and is being evaluated as a treatment for human metastatic neuroendocrine cancers. Enteroviruses are responsible for the important human disease poliomyelitis. Equine rhinovirus 1 has been assigned to the genus Aphthovirus and renamed equine rhinitis A virus. Figure 52.1 Negative contrast electron micrograph of poliovirus particles. The bar represents 100 nm. Reprinted with permission: Fauquet, C.M., et al. (Eds.), 2005. Virus Taxonomy Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, pp. 757. In general, picornaviruses infect only one or a very small number of host species. However, foot-and-mouth disease virus and encephalomyocarditis virus infect a very wide host range. Horizontal transmission occurs, usually by faecal–oral, fomite or airborne routes. Persistent infections occur with a number of species, notably foot-and-mouth disease virus (Bergmann et al. 1996, Mezencio et al. 1999) and swine vesicular disease virus (Lin et al. 1998). The mechanism of persistence is unclear but may be due to antigenic variation (Woodbury 1995). Genetic recombination, complementation and phenotypic mixing have been described. Mixed infections in individual animals, particularly the African buffalo, with different serotypes of foot-and-mouth disease virus are known to occur. Infections with porcine teschoviruses (PTVs) are widespread in pig herds but usually subclinical. Certain PTV serotypes (PTV-1, PTV-2, PTV-3, PTV5, PTV-6) are associated with a range of clinical conditions including SMEDI, encephalomyelitis, diarrhoea, pneumonia and heart disease. Picornaviruses of animals are summarized in Table 52.1. Cattle, sheep, goats, pigs and buffalo are susceptible to FMD. In addition several wildlife species including African buffalo, elephants, hedgehogs, deer and antelope are susceptible. Large amounts of virus are shed by infected animals in all secretions and excretions. Virus shedding begins during the incubation period, usually beginning about 24 hours before the appearance of clinical signs, and infectivity of animals is much reduced by four to five days after the lesions develop. Transmission occurs by direct contact, by animal products including meat, offal, milk, semen and embryos, by the airborne route or by mechanical carriage by people, vehicles and fomites. Infected groups of animals, particularly pigs, shed large quantities of virus in exhaled air as an aerosol. Under suitable conditions of low temperature, high humidity and moderate winds such aerosols may spread the virus over long distances. Typically spread over land is within 10 km of the source. However, turbulence is generally less over water than over land and in 1981 the disease spread over 200 km across the English Channel from France to the south coast of England. Cattle are most susceptible to infection because of their large respiratory volume and the low infective dose required. The virus is moderately resistant but is sensitive to pH outside the range 6.0 to 9.0. Virus can remain infective on soil for three days in the summer and for 28 days in the winter. Following death and rigor mortis, the production of lactic acid in muscle inactivates the pH-labile virus but virus may persist in offal and bone marrow. Foot-and-mouth disease virus can persist in the pharyngeal region of animals that have recovered from FMD or in vaccinated animals following contact with live virus. Persistence can last up to three years in cattle, several months in sheep and up to five years in African Cape buffalo. It is unclear if persistence occurs in pigs (Bergmann et al. 1996, Mezencio et al. 1999). The transmission of infection from carrier to susceptible animals has only been demonstrated satisfactorily for African Cape buffalo. • Diagnosis is based on the demonstration of FMD viral antigen in samples of tissue or fluid. Samples may be tested using ELISA or CFT. The indirect sandwich ELISA (Roeder & Le Blanc Smith 1987) is the preferred test as it is more sensitive and specific and is available in kit form. • The reverse transcription polymerase chain reaction (RT-PCR) is suitable for the amplification of genome fragments of FMDV in diagnostic material (Amarel-Doel et al. 1993, Bastos 1998, Reid et al. 2000). Sensitive real time RT-PCR protocols are also available (Reid et al. 2001, Alexandersen et al. 2002, Reid et al. 2002, King et al. 2006). Efforts are being made to adapt the methodology for use in the field (King et al. 2008). Nucleotide sequencing of the VP1 gene, which encodes a capsid protein, of an isolate from an outbreak of FMD is used to provide a means of comparison with other isolates of the same serotype in order to determine the possible origin of the outbreak. In addition, the study of VP1-based phylogenies has revealed that different genotypes within the O and SAT types evolve in discrete geographical regions, the resulting variants are known as topotypes. • Virus isolation can be carried out in sensitive cell lines such as primary bovine thyroid or kidney cells. Established cell lines such as IB-RS-2 and BHK-2 may be used but are less sensitive than primary cells. The epithelium sample should be blotted dry to remove most of the glycerol, which is toxic to cells, and homogenized to form a suspension in a small volume of tissue culture medium. Following clarification the suspension is inoculated onto cell culture and examined over 48 hours for evidence of cytopathic effect (CPE). In the event of failure to detect CPE a second passage should be carried out and examined for a further 48 hours. • Demonstration of specific antibody can also be used to confirm a diagnosis but generally requires absence of vaccination. Interpretation of antibody titres in enzootic areas may be difficult due to the possibility of previous infection. Suitable serological tests include virus neutralization and liquid-phase blocking or competitive ELISA (Hamblin et al. 1986, Van Maanen 1990). These assays detect antibodies to structural proteins and are therefore serotype-specific. The detection of antibodies to non-structural proteins (NSPs) by ELISA or immunoblotting has been shown to be useful in detecting animals currently or previously infected regardless of whether the animal has been vaccinated (De Diego et al., 1997, Bergmann et al. 2000, Paton et al. 2006). The polyproteins 3AB or 3ABC are generally considered the most useful NSP test antigens and are produced by recombinant techniques (Mackay et al. 1997, Sorensen et al. 1998). It is important that the FMD vaccines used do not contain even trace amounts of NSPs. Some infected animals may not be detected using NSP antibody assays (Mackay 1998) and therefore these assays are probably best used on a herd rather than an individual animal basis. The NSPs are highly conserved and are not serotype-specific.
Picornaviridae
Foot-and-mouth disease
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Picornaviridae
Only gold members can continue reading. Log In or Register to continue