Chapter 9 PHEOCHROMOCYTOMA AND MULTIPLE ENDOCRINE NEOPLASIA
PHEOCHROMOCYTOMA
Pheochromocytoma is a catecholamine-secreting tumor arising from the adrenal medulla. Historically, pheochromocytoma was most commonly identified as an incidental finding at necropsy; an antemortem diagnosis was uncommon in dogs and rare in cats (Schaer, 1980; Twedt and Wheeler, 1984; Bouayad et al, 1987; Henry et al, 1993; Gilson et al, 1994). This was due, in part, to a low index of suspicion for pheochromocytoma by veterinarians, the often vague and episodic nature of the clinical signs which were frequently attributed to other more common disorders, and the lack of a good diagnostic screening test for the disease. With the establishment of abdominal ultrasound as part of the routine diagnostic evaluation of the ill dog or cat, the identification of adrenal masses and consequently the consideration of pheochromocytoma has increased tremendously during the past decade. To illustrate this point, pheochromocytoma was suspected antemortem in only 11 confirmed cases seen at our hospital between 1984 and 1994 but was suspected antemortem in 33 confirmed cases between 1996 and 2002.
Etiology
The endocrine cells of the adrenal medulla are called chromaffin cells. They are derived from the neuroectoderm and are capable of synthesizing, storing, and secreting catecholamines (epinephrine and norepinephrine). Although most chromaffin cells are in the adrenal medulla, small numbers of extra-adrenal chromaffin cells also exist in and about sympathetic ganglia. The function of the extra-adrenal chromaffin cells is not known. Most regress early in postnatal development, although remnants may become the site of subsequent tumor formation (Young and Landsberg, 1998).
Pheochromocytoma is a catecholamine-producing tumor derived from the chromaffin cells. Pheochromocytoma arising from the chromaffin cells in the adrenal medulla is most common, may occur in one or both adrenal glands, and may be part of the multiple endocrine neoplasia syndrome (see page 459). Approximately 10% of catecholamine-secreting tumors in humans arise from extra-adrenal chromaffin cells and are called paragangliomas. Paragangliomas are located primarily within the abdomen but may also be found in the heart, the posterior mediastinum, along the aorta, in and around the kidney, and the urinary bladder wall (Goldfien, 2001; Young and Landsberg, 1998). Paragangliomas are rare in the dog and cat (Patnaik et al, 1990; Hines et al, 1993; Barthez et al, 1997; Buchanan et al, 1998).
Pheochromocytomas are usually solitary, slow-growing, vascular tumors ranging in size from nodules of less than 0.5 cm in diameter to masses greater than 10 cm in diameter. Pheochromocytomas affecting both adrenal glands and pheochromocytoma with an adrenocortical tumor in the contralateral adrenal gland have also been reported (Bouayad et al, 1987; Gilson et al, 1994; von Dehn et al, 1995; Bennett and Norman, 1998). Pheochromocytomas should be considered a malignant tumor in dogs (Bouayad et al, 1987). Approximately 40% of our dogs with pheochromocytoma had invasion or extension of the tumor into the lumen of the adjacent vena cava or phrenicoabdominal vein and/or entrapment and compression of the caudal vena cava. Mural invasion and/or luminal narrowing of the aorta, renal vessels, adrenal vessels, and hepatic veins may also occur (Bouayad et al, 1987). Approximately 30% of our dogs had metastasis of the tumor at the time of necropsy. Distant sites of metastasis include the liver, lung, regional lymph nodes, spleen, heart, kidney, bone, pancreas, CNS, and spinal canal (Gilson et al, 1994; Barthez et al, 1997).
Gross and histologic differentiation of pheochromocytoma from adrenocortical neoplasia can be difficult, especially with large tumors. Immunohistochemical staining for chromogranin A and synaptophysin can be used to determine the site of origin (i.e., adrenal cortex vs medulla) of the tumor; techniques which are routinely performed on suspected adrenal medullary tumors in our hospital. Synaptophysin is a membrane component of synaptic vesicles in neurons and neuroendocrine cells. Chromogranin A is a protein present in the secretory granules of endocrine cells and functions in hormone packaging, secretory granule stabilization, and regulation of hormone secretion (Winkler and Fischer-Colbrie, 1992). Chromogranin A is a major constituent of secretory granules of the adrenal medulla, pituitary, parathyroid, thyroid C cells, pancreatic islets, endocrine cells of the gastrointestinal tract, and sympathetic nerves (Doss et al, 1998). Chromogranin A is not present in the cells of the adrenal cortex. In dogs, tumors with the strongest and most consistently positive chromogranin A staining are the pheochromocytoma and chemodectoma. Parathyroid, pituitary, and islet cell tumors yield inconsistent results. Endocrine tumors that produce chromogranin A may also co-secrete immunoreactive chromogranin A into the circulation, along with their characteristic hormone (Deftos et al, 1989). Documentation of increased concentrations of plasma chromogranin A has been proposed as a screening test to identify peptide-producing endocrine neoplasms in humans (O’Connor and Deftos, 1986; Hsiao et al, 1991), and changes in plasma chromogranin A concentrations have been used as a marker for treatment response (Moattari et al, 1989). Measurement of circulating chromogranin A concentration as a means to identify pheochromocytoma has not yet been reported in dogs or cats.
Pathophysiology
Pheochromocytoma is a tumor of the sympathetic nervous system. Preganglionic fibers of the sympathetic nervous system are cholinergic and liberate acetylcholine as their neurotransmitter. Postganglionic fibers are adrenergic and liberate norepinephrine and dopamine. The adrenal medulla can be viewed as sympathetic postganglionic neurons without axons. Normally, norepinephrine and epinephrine are both secreted by the adrenal medulla. However, the normal adrenal gland harbors high concentrations of the N-methylating enzyme, which converts norepinephrine to epinephrine, so that epinephrine is the predominant catecholamine produced by the adrenal medulla (Werbel and Ober, 1995). In cats, dogs, and humans, 60%, 70%, and 80% of catecholamine output from the adrenal medulla is epinephrine, respectively (Goldfien, 2001). Circulating norepinephrine is also derived from autonomic nerve endings. In contrast, most pheochromocytomas in humans contain predominantly norepinephrine or a mixture of norepinephrine, epinephrine, and dopamine (Prys-Roberts, 2000). Tumors rarely produce epinephrine exclusively (Young and Landsberg, 1998). Paragangliomas usually secrete norepinephrine. Evaluation of catecholamine secretory patterns for pheochromocytoma in the dog and cat have not been reported.
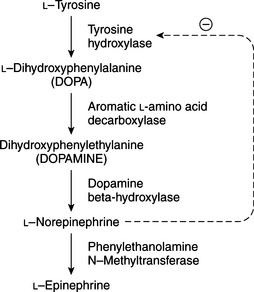
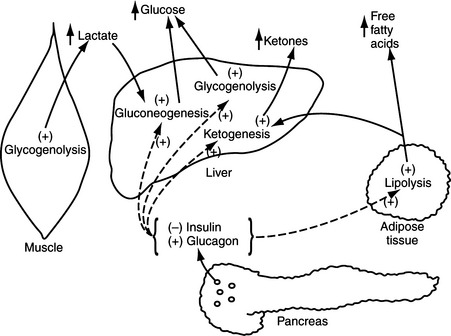
Although the biosynthesis and storage of catecholamines in pheochromocytomas may be different than in the normal adrenal medulla, chromaffin granules from pheochromocytomas are morphologically, physically, and functionally similar to chromaffin granules of the adrenal medulla (Johnson et al, 1982; Roizen et al, 1984). The increase in tissue turnover of catecholamines in vitro and in vivo in some tumors suggests an alteration in the regulation of catecholamine biosynthesis, possibly because of an impairment in feedback inhibition of tyrosine hydroxylase. The primary rate-limiting step in the synthesis of catecholamines is the hydroxylation of tyrosine by the enzyme tyrosine hydroxylase (Fig. 9-1). Cytoplasmic concentrations of norepinephrine normally act directly to inhibit the activity of tyrosine hydroxylase and thereby control catecholamine production (Young and Landsberg, 1998). With sympathetic nerve stimulation, the negative feedback inhibition of norepinephrine on tyrosine hydroxylase is removed, as newly formed and stored norepinephrine is released from the cell into the extracellular space. Increased enzyme activity then promotes further norepinephrine synthesis. In pheochromocytomas, ordinary intracellular concentrations of norepinephrine do not inhibit tyrosine hydroxylase. In addition, the turnover rate of catecholamines may be markedly increased, thereby preventing negative feedback inhibition of tyrosine hydroxylase.
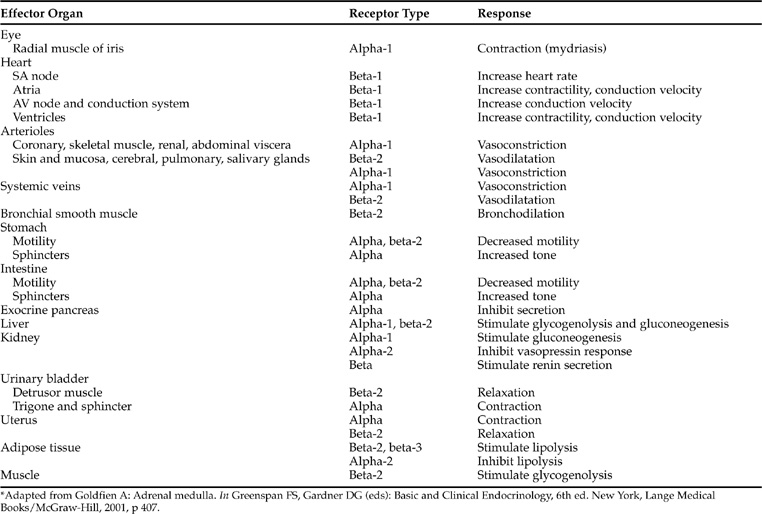
The clinical manifestations of pheochromocytoma are predictable from the known physiologic and pharmacologic effects of catecholamines (Tables 9-1 and 9-2). The catecholamines epinephrine, norepinephrine, and dopamine exert their physiologic effects by interactions with appropriate receptors at the target tissue. Two classes of receptors (alpha [α] and beta [β]) exist which are capable of responding to epinephrine and norepinephrine. α-receptors are further subdivided into α1 and α2 receptors, and β-receptors into β1-, β2-, and β3-receptors. α1-receptors mediate a wide variety of effects, most prominently involving smooth muscle and including vasoconstriction, intestinal relaxation, uterine contraction, and pu pillary dilation (Young and Landsberg, 1998; Goldfien, 2001). α2-receptors are located on presynaptic sympathetic neurons, on cholinergic neurons within the gut, on CNS neurons involved in the regulation of cardiovascular function, on platelets, and on blood vessels. The α2-receptor mediates inhibition of norepinephrine release from adrenergic neurons, inhibition of acetylcholine release from cholinergic neurons, potentiation of the baroreceptor vasodepressor response mediated through central regulatory neurons, platelet aggregation, and vasoconstriction. β-receptors primarily mediate cardiac stimulation, bronchodilatation, and vasodilatation. The β1-receptor mediates cardiac stimulation and lipolysis; the β2-receptor mediates bronchodilatation, vasodilatation, and prejunctional stimulation of norepinephrine release from sympathetic neurons; and the β3-receptors primarily regulate energy expenditure and lipolysis. The mode of action of catecholamines is related to their potency at specific receptors. Epinephrine and norepinephrine are considered equipotent at sites with α1-, α2-, and β1-receptors, whereas β2-receptors are stimulated more by epinephrine than by norepinephrine and β3-receptors are stimulated more by norepinephrine than by epinephrine. Tissue response to catecholamines is dependent on the number and type of catecholamine receptors present on the cell membrane, and the proportion of α- and β-receptors and their respective thresholds for response in the particular tissue in question (Tables 9-1 and 9-2). For example, the smooth muscle surrounding blood vessels contains both α1- and β2-receptors. α1-receptors predominate when elevated plasma concentrations of catecholamines are present, resulting in vasoconstriction and the development of hypertension, a common problem associated with pheochromocytoma.
Catecholamine action is terminated by active reuptake of catecholamines by nerve endings or metabolic transformation of the hormone into inactive metabolites which are excreted by the kidney. Two hepatic enzyme systems, monoamine oxidase and catechol-O-methyl transferase metabolize norepinephrine and epinephrine into metanephrine, normetanephrine, and finally vanillylmandelic acid (Young and Landsberg, 1998). These urinary metabolites reflect the amount of catecholamines released by adrenergic and pheochromocytoma tissue.
Signalment
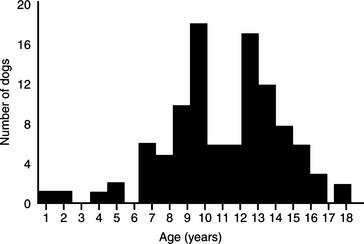
Pheochromocytoma has been identified most commonly in older dogs. The mean age of 98 dogs with pheochromocytoma seen at our hospital was 11 years, with a range of 1 to 18 years (Fig. 9-2). There is no apparent sex predisposition. Fifty-seven of our dogs were male or male castrate and 41 were female or female spayed. There also does not appear to be a breed predisposition, although the Miniature Poodle, German Shepherd dog, Boxer, Golden Retriever, Labrador Retriever, Doberman Pinscher, and Sheltie were the most frequently affected breeds in our group of dogs (Table 9-3). However, the higher frequency in these breeds may merely represent their current popularity in our area of the country.
TABLE 9-3 BREED DISTRIBUTION AMONG 98 DOGS WITH PHEOCHROMOCYTOMA
| Breed | Number of Dogs |
|---|---|
| Miniature Poodle | 10 |
| German Shepherd dog | 10 |
| Boxer | 7 |
| Golden Retriever | 6 |
| Labrador Retriever | 5 |
| Doberman Pinscher | 5 |
| Shetland Sheepdog | 5 |
| Mixed Breed | 5 |
| Miniature Schnauzer | 4 |
| Dachshund | 3 |
| Great Dane | 3 |
| Siberian Husky | 2 |
| Chow Chow | 2 |
| Dalmatian | 2 |
| Rottweiler | 2 |
| Springer Spaniel | 2 |
| Weimeraner | 2 |
| Other (1 each) | 23 |
An adrenal pheochromocytoma has been reported in an 11-year-old female domestic shorthair cat (Henry et al, 1993) and was an incidental finding on abdominal ultrasound in a 15-year-old domestic shorthair cat with concurrent apocrine gland adenocarcinoma (Chun et al, 1997). An extra-adrenal pheochromocytoma (paraganglioma) has been reported in an 18-year-old spayed domestic shorthair cat (Patnaik et al, 1990). The paraganglioma was located close to but not involving the left adrenal gland, and was closely adhered to the renal capsule of the left kidney.
Clinical Manifestations
Clinical signs and physical examination findings develop as a result of the space-occupying nature of the tumor and its metastatic lesions or as a result of the excessive secretion of catecholamines (Table 9-4). The most common clinical signs are generalized weakness and episodic collapse. Additional signs that may be observed by the owner or be evident during the physical examination include intermittent agitation, pacing, excessive panting, tachypnea, a ‘pounding’ heart, polyuria, and polydipsia. Polyuria and polydipsia may be caused by excessive catecholamine secretion or develop secondary to renal failure caused by tumor thrombus-induced interference with renal blood flow. Excess catecholamine secretion may also cause severe systemic hypertension, which can result in sudden blindness due to retinal hemorrhage and detachment, clinical signs resulting from spontaneous hemorrhage into the retroperitoneal space, abdominal cavity or an organ, most notably neurologic signs as a result of cerebrovascular hemorrhage, or bleeding from the nasal or oral cavity (Gilson et al, 1994; Williams et al, 2001; Whittemore et al, 2001).
TABLE 9-4 FREQUENCY OF CLINICAL SIGNS IN 40 DOGS WITH PHEOCHROMOCYTOMA AND IDENTIFICATION OF NO OTHER CONCURRENT DISORDER
| Clinical Sign | Number of Dogs | Percentage of Dogs |
|---|---|---|
| Collapsing episodes* | 13 | 33% |
| Weakness* | 12 | 30% |
| Panting/tachypnea* | 12 | 30% |
| Polyuria/polydipsia | 10 | 25% |
| Lethargy | 10 | 25% |
| Vomiting | 9 | 23% |
| Inappetence | 8 | 20% |
| Anxiety/agitation/pacing* | 6 | 15% |
| Diarrhea | 4 | 10% |
| Abdominal distention | 4 | 10% |
| Hemorrhage (nasal, ocular, gingival) | 4 | 10% |
| Acute blindness | 3 | 8% |
| Tremors* | 3 | 8% |
| Weight loss | 3 | 8% |
| Tachycardia/“pounding” heart* | 2 | 5% |
| Rear limb edema | 2 | 5% |
| Tender or painful abdomen | 2 | 5% |
| Adipsia | 1 | 3% |
| No clinical signs | 4 | 10% |
* Usually reported to be intermittent by the owner.
Catecholamine secretion is sporadic and unpredictable. As such, clinical manifestations and systemic hypertension tend to be paroxysmal and are usually not evident at the time the dog is examined. Because clinical signs are often vague, nonspecific, and easily associated with other disorders, pheochromocytoma is often not considered a possible differential diagnosis until an adrenal mass is identified with abdominal ultrasound (see Incidental Adrenal Mass, page 458). Pheochromocytoma may also be an unexpected or incidental finding at necropsy, may result in acute collapse and death from a sudden, massive, and sustained release of catecholamines by the tumor, or may cause periodic clinical signs (e.g., collapsing episodes, tachypnea, pounding heart rate) which strongly suggest pheochromocytoma at the time of initial examination.
A retrospective evaluation of 98 dogs with pheochromocytoma seen at our hospital revealed that pheochromocytoma was an incidental finding at necropsy in 22% of the dogs, was an unexpected finding at necropsy in 33% of dogs that were euthanized because of concurrent disease, resulted in acute death in 14% of dogs, was an unexpected finding during abdominal ultrasound or anesthesia (i.e., sudden surge in blood pressure) in 5% of dogs, and was the primary differential diagnosis in 26% of the 98 dogs. These later dogs had clinical manifestations (e.g., intermittent collapsing episodes, agitation, pacing, severe tachycardia, tachypnea) that strongly suggested pheochromocytoma at the time of initial examination. The suspicion for pheochromocytoma was further enhanced by identifying an adrenal mass with abdominal ultrasound.
Unfortunately, many of the clinical signs caused by pheochromocytoma are vague and usually associated with other, more common disorders. In addition, pheochromocytoma typically occurs in conjunction with other, often serious disorders (Table 9-5). Clinicians tend to focus on the more recognizable disorders and often overlook the possibility of concurrent pheochromocytoma; an oversight which can have catastrophic consequences.
TABLE 9-5 CONCURRENT DISORDERS IDENTIFIED IN 58 OF 98 DOGS WITH PHEOCHROMOCYTOMA
| Generalized Disorders | Neoplasms |
|---|---|
* Consistent with multiple endocrine neoplasia (MEN) seen in humans.
There may be a correlation between the size of a pheochromocytoma and the presence or severity of clinical signs (Bouayad et al, 1987). In our experience, small, well-demarcated pheochromocytomas, often causing minimal enlargement of the adrenal gland, are more commonly identified as incidental findings during an abdominal ultrasound or at necropsy. In contrast, dogs with clinical signs suggestive of pheochromocytoma typically have an easily identified adrenal mass, often with concurrent compression or invasion of surrounding blood vessels. Pheochromocytoma was an unexpected finding during abdominal ultrasound or at necropsy in 11 of our recently diagnosed 54 dogs. In 9 (82%) of these 11 dogs, the pheochromocytoma was either not identified during abdominal ultrasound or was a small (<1.5 cm diameter), well-demarcated mass. In contrast, only 8 (19%) of 43 dogs with clinical signs consistent with pheochromocytoma had a mass less than 2.0 cm in diameter on abdominal ultrasound. Thirty-five of 43 dogs with clinical signs had an easily recognizable, large adrenal mass which was distorting the adrenal gland, often compressing or invading surrounding structures.
Physical Examination
Physical examination findings in dogs with pheochromocytoma are quite variable and dependent, in part, on the secretory activity of the tumor at the time of the examination, the size of the tumor, and the presence of concurrent problems. In our experience, the physical examination is often unremarkable and any abnormalities identified are more likely related to the older age of the dog (e.g., dental disease, lipomas, low-grade mitral murmurs) than to the presence of a pheochromocytoma. When identified, abnormalities are usually a result of excessive catecholamine secretion by the tumor and typically involve the respiratory (i.e., tachypnea, excessive panting), cardiovascular (i.e., tachycardia, cardiac arrhythmias, weak femoral pulses), and musculoskeletal systems (i.e., weakness, muscle wasting) (Table 9-6). Cardiac arrhythmias include premature ventricular contractions, third degree heart block, and atrial tachycardia.
TABLE 9-6 FREQUENCY OF ABNORMAL FINDINGS ON PHYSICAL EXAMINATION OF 40 DOGS WITH PHEOCHROMOCYTOMA AND IDENTIFICATION OF NO OTHER CONCURRENT DISORDER*
| Physical Finding | Number of Dogs | Percentage of Dogs |
|---|---|---|
| Panting, tachypnea | 15 | 38% |
| Weakness | 9 | 23% |
| Tachycardia | 7 | 18% |
| Thin, muscle wasting | 6 | 15% |
| Cardiac arrhythmias | 6 | 15% |
| Hemorrhage (nasal, gingival, ocular) | 6 | 15% |
| Weak pulses | 4 | 10% |
| Pale mucous membranes | 4 | 10% |
| Abdominal pain | 4 | 10% |
| Ascites | 4 | 10% |
| Lethargy | 3 | 8% |
| Palpable abdominal mass | 2 | 5% |
| Shocky | 2 | 5% |
| Blindness, retinal detachment | 2 | 5% |
| Rear limb edema | 1 | 3% |
| Shaking, muscle tremors | 1 | 3% |
| Lateral recumbency | 1 | 3% |
| Anterior uveitis | 1 | 3% |
| Unremarkable | 15 | 38% |
* Excluding common geriatric findings such as lipomas, dental disease, and incidental cardiac murmurs.
An abdominal mass was palpable in only 5%, peripheral edema or ascites was present in 10%, and pain on palpation of the abdomen was identified in 10% of 40 dogs. None of the dogs with abdominal pain had a palpable abdominal mass. Fluid retention most likely results from local invasion and obstruction of the caudal vena cava by the tumor, a phenomenon that was documented at surgery or necropsy in 40% of our 98 dogs and in each of the dogs with ascites or peripheral edema (Schoeman and Stidworthy, 2001). Obstruction of the posterior vena cava may also cause distention of the superficial veins of the ventral abdominal wall (Twedt and Wheeler, 1984).
Mydriasis, retinal hemorrhage, retinal detachment, and blindness may develop following sustained severe hypertension, although these were infrequent findings in our dogs. Interestingly, 2 dogs had persistent mild epistaxis, 2 dogs had persistent mild hemorrhage from sites of minor surgery performed within a week of being brought to our hospital, and 1 dog had persistent mild gingival bleeding; problems which were presumably a result of hypertension. Neurologic signs, including seizures, head tilt, nystagmus, and strabismus, have also been reported (Twedt and Wheeler, 1984; Gilson et al, 1994). Neurologic signs (spinal pain, rear limb ataxia and paresis) have also been described in 4 dogs with metastatic extra-adrenal paraganglioma (Hines et al, 1993) and 2 dogs with metastatic pheochromocytoma (Platt et al, 1998).
Clinical Pathology
There are no consistent abnormalities in the CBC, serum biochemical panel, or urinalysis which would raise suspicion of pheochromocytoma. There were no abnormalities identified on the CBC and serum biochemical panel in approximately 30% of our dogs with pheochromocytoma and no significant concurrent disease. Failure to identify abnormalities on routine bloodwork is helpful in differentiating a catecholamine-secreting from a cortisol- or aldosterone-secreting adrenal tumor (see page 458). Mild thrombocytopenia, typically ranging from 150,000 to 200,000/μL, was the most consistent abnormality on a CBC, identified in approximately 20% of our dogs. There was no correlation between identification of hemorrhage on the physical examination and thrombocytopenia on the CBC. A mild non-regenerative anemia (PCV, 25 to 35%), mild hemoconcentration (PCV, 45 to 55%), and neutrophilic leukocytosis were identified in less than 10% of our dogs. The cause and effect relationship between pheochromocytoma and these findings is not known. The non-regenerative anemia may be an anemia associated with chronic disease or be a result of other concurrent disease. The increase in the hematocrit most likely is a reflection of a decrease in plasma volume but could also result from either catecholamine-stimulated erythropoietin release from the kidney or an erythropoietin-like peptide produced and secreted by the pheochromocytoma (Young and Landsberg, 1998).
An increase in serum alkaline phosphatase and alanine aminotransferase activity, hypoalbuminemia, hypercholesterolemia, and azotemia are the most common abnormalities on the serum biochemical panel, being identified in approximately 80%, 60%, 45%, 25%, and 25% of 61 dogs in one retrospective study (Barthez et al, 1997). However, when results of the serum biochemistry panel are evaluated in dogs with pheochromocytoma and no significant concurrent disease, the prevalence of these abnormalities decreases to 40%, 10%, 5%, 15%, and 10%, respectively. Unfortunately, abnormalities identified on the serum biochemical panel are not helpful in establishing a diagnosis of pheochromocytoma. In our experience, there does not appear to be any correlation between changes in serum liver enzyme activities and metastatic disease within the liver. Elevations in liver enzymes were not present in most of our dogs with necropsy-confirmed hepatic metastasis of a pheochromocytoma.
The blood glucose concentration was between 120 mg/dl and 180 mg/dl and the urine was negative for the presence of glucose in approximately 20% of our dogs with pheochromocytoma, a finding reported to occur by others (Twedt and Wheeler, 1984; Barthez et al, 1997). Carbohydrate intolerance and elevated fasting blood glucose concentrations have been reported in humans with pheochromocytoma (Young and Landsberg, 1998). Epinephrine has both direct and indirect effects to stimulate hepatic glycogenolysis and hepatic and renal gluconeogenesis; provide muscle tissue with an alternative source of fuel by mobilizing muscle glycogen and stimulating lipolysis; mobilize gluconeogenic precursors (e.g., lactate, alanine, and glycerol); and inhibit glucose utilization by insulin-sensitive tissues like skeletal muscle (Fig. 9-3) (Cryer, 1993; Karam, 2001). The carbohydrate intolerance that develops in humans with pheochromocytoma is usually mild and does not require therapy. Interestingly, 3 of our 98 dogs with pheochromocytoma had concurrent insulin-dependent diabetes mellitus and 2 of these dogs developed severe diabetic ketoacidosis. In one dog, the diabetic state was well-controlled at the time pheochromocytoma was diagnosed. What role, if any, catecholamine-induced insulin antagonism may have played in the development of diabetes or ketoacidosis in these dogs is not known. It is possible that our dogs with carbohydrate intolerance and clinical diabetes were in a subclinical diabetic state (i.e., islet pathology and decreased beta cell numbers) at the time the pheochromocytoma developed. Catecholamine-induced insulin resistance increased the demand for insulin secretion; a demand which could not be adequately met in dogs with impaired insulin secretory capabilities. The severity of the ensuing hyperglycemia would be dependent on the severity of the islet pathology and the insulin resistance. We always include pheochromocytoma in the differential diagnoses for causes of poor glycemic control and ketoacidosis in diabetic dogs and cats.
The only consistent abnormality identified in the urinalysis was proteinuria, which was identified in approximately 15% of our dogs with pheochromocytoma and no significant concurrent disease. Mean urine specific gravity was 1.022, with a range of 1.004 to 1.041. Identification of hyposthenuria and isosthenuria in some dogs supports owner’s observations of polydipsia and polyuria in their dogs (see Table 9-4). Catecholamines, most notably norepinephrine, inhibit pituitary vasopressin secretion via a nonpressor interaction with arterial baroreceptors (Young and Landsberg, 1998). Hemodynamic factors and catecholamine-induced inhibition of vasopressin responses in the renal collecting tubules may also contribute to the development of polyuria and polydipsia (Krothapalli et al, 1983).
Diagnostic Imaging Techniques
ABDOMINAL ULTRASOUND.
Abdominal ultrasound plays a critical role in the diagnosis of pheochromocytoma. Clinical signs of pheochromocytoma are episodic and often vague, findings on physical examination are usually non-contributory, and the clinician’s index of suspicion for the disease is usually low. Pheochromocytoma is often considered only after an adrenal mass is identified on abdominal ultrasound performed as part of the routine diagnostic evaluation of any sick dog or cat. Ultrasound is an effective diagnostic tool for identifying an adrenal mass. Once identified, the clinician can then review the history, physical findings, and initial blood tests and perform the necessary diagnostic tests to determine the functional status of the mass and its most likely origin (i.e., cortex or medulla; see Incidental Adrenal Mass, page 458).
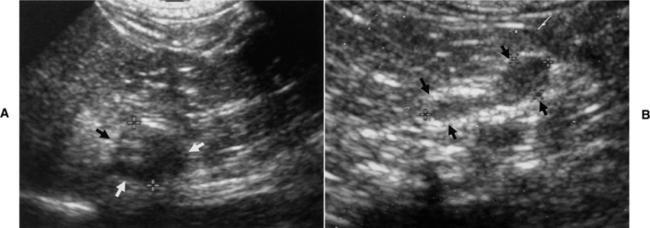
The most common ultrasound finding with pheochromocytoma is adrenomegaly (i.e., an adrenal mass) with a normal-sized contra lateral adrenal gland (Fig. 9-4). In contrast, dogs with adrenal-dependent hyperadrenocorticism should have unilateral adrenomegaly and a decreased size (i.e., atrophy) of the contralateral adrenal gland (Fig. 9-5) (see Chapter 6). An obvious adrenal mass was identified in approximately 85% of our dogs with pheochromocytoma, and the majority of the remaining dogs had asymmetry in adrenal gland size with a mass suspected in the larger adrenal gland. Pheochromocytoma may develop in both adrenal glands, resulting in bilateral adrenal masses (see Fig. 9-8) (Gilson et al, 1994; Barthez et al, 1997). Pheochromocytoma and an adrenocortical tumor can also occur simultaneously, which can pose a difficult diagnostic and therapeutic challenge (von Dehn et al, 1995; Bennett and Norman, 1998). Although finding normal-sized adrenal glands does not rule out pheochromocytoma, in our experience, there is a direct correlation between size of the tumor and severity of clinical signs and small pheochromocytomas (i.e., less than 1 cm in size) are usually incidental findings at necropsy.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree