Chapter 18 RENAL HORMONES AND ATRIAL NATRIURETIC HORMONE
THE KIDNEY
Hormones Synthesized by the Kidneys
The kidneys are involved in four major hormonal systems in the body. First, they secrete renin, a proteolytic enzyme that initiates a chain of events leading to production of the circulating peptide angiotensin II, which plays an important role in the regulation of salt and water metabolism, stimulates synthesis and secretion of aldosterone, and is a regulator of blood pressure. Second, the kidneys produce kinins, which are thought to enhance the effects of aldosterone and vasopressin in the kidneys. Kidney disease and renal hormones are frequently involved in the pathogenesis of hypertension. Third, the kidneys secrete erythropoietin, a glycoprotein that helps to control red cell production. Fourth, the final step in activation of vitamin D occurs in the kidneys. The kidneys remove 25-hydroxycholecalciferol from the bloodstream and hydroxylate it to 1,25-dihydroxycholecalciferol. This agent increases the plasma calcium concentration by increasing its intestinal absorption, enhancing renal tubular resorption, and stimulating bone resorption. Vitamin D and its actions are discussed thoroughly in Chapters 16 and 17.
Erythropoietin
PHYSIOLOGY.
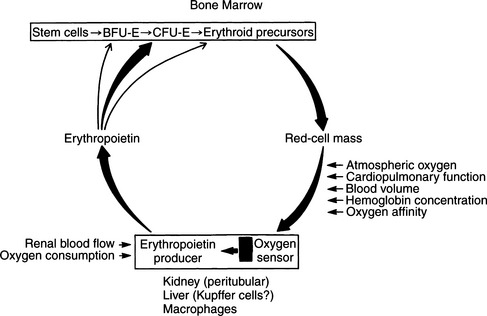
It has long been realized that hypoxia is a potent stimulus to red cell production. In human subjects, within a few hours of exposure to hypoxia, the reticulocyte count increases, denoting the presence of new red blood cells in the circulation; this is followed by an increase in the circulating red cell mass. This response is the result of the increased production of a glycoprotein, erythropoietin. The kidney is the major source of this hormone, although it is also produced in the liver, particularly in fetuses. Day-to-day production of erythropoietin depends on the partial pressure of oxygen (PO2) of blood perfusing the kidneys. The target cells for erythropoietin are the colony-forming units–erythroid (CFU-E) cells in the bone marrow. Erythropoietin reacts with receptors on CFU-E cells, leading to increased production of proerythroblasts and release of more erythrocytes into the circulation (Fig. 18-1) (Erslev, 1991).
BENEFITS OF SYNTHETIC ERYTHROPOIETIN.
Human erythropoietin has been cloned and represents one of the most successful products of recombinant deoxyribonucleic acid (DNA) technology. Recombinant human erythropoietin (rHuEPO; epoetin) is available for therapeutic use and has revolutionized the treatment of anemia in humans with chronic renal failure. Epoetin is virtually identical to human urinary erythropoietin; it has the same amino acid sequence but a slightly different glycosylation pattern. Most treated humans have reported dramatic improvement in their quality of life and sense of well-being as a result of correction of anemia. The benefits of improved exercise tolerance, appetite, strength, and cognitive abilities without the need for blood transfusions outweigh some of the risks associated with the use of rHuEPO.
Most experience with the use of rHuEPO in animals has been with epoetin-α (Epogen). Epogen is suspended in human albumin to prevent adherence to glass and does not contain a preservative. The structure of erythropoietin is well conserved across species lines. Both dogs and cats respond appropriately to administration of this synthetic hormone. When given to dogs or cats with chronic renal failure, rHuEPO causes a dose-dependent increase in the hematocrit and corrects anemia (Cowgill, 1990, 1995). A transient, moderate reticulocytosis is initially observed within the first week of therapy in most animals. The bone marrow myeloid:erythroid ratio decreases, illustrating the increased erythropoietic response. Some animals have a transient thrombocytosis during therapy. This response may be seen in some human patients as well, and it is not known whether this is a direct effect of the rHuEPO on megakaryocytes or a secondary effect caused by iron deficiency (Polzin et al, 1995). Correction of the hematocrit to low normal takes about 2 to 8 weeks, depending on the measurement when treatment was initiated and the dose administered. Most clients observe improved clinical status in their pets as the hematocrit increases. These changes include improved appetite, body weight, energy, and sociability (Cowgill, 1990).
DOSAGE AND ROUTE OF RHUEPO ADMINISTRATION.
Both intravenous and subcutaneous routes of administration of rHuEPO have been effective, and no difference has been seen in the percentage of patients that develop antibodies. Plasma concentrations persist longer after subcutaneous administration, which allows lower total doses to be given. Definitive dosing schedules are continuing to evolve. Currently, starting doses of 50 to 150 U/kg given subcutaneously three times per week are recommended (Polzin et al, 1995). Weekly monitoring should continue until a target hematocrit is achieved; that is, a packed cell volume (PCV) of 33% to 40% in dogs and 30% to 35% in cats. If a dog or cat has severe anemia (PCV <14%) but does not require transfusion, daily therapy with 150 U/kg may be preferred for the first week. In hypertensive animals or if the anemia is not severe, 50 U/kg three times weekly may prevent progressive increases in blood pressure or iron-deficient erythropoiesis.
ADVERSE REACTIONS TO RHUEPO
Identified Adverse Reactions.
A variety of adverse reactions to rHuEPO therapy have been identified, although a direct causal relationship between drug and side effect may not always be established. Adverse reactions include refractory anemia, polycythemia, vomiting, seizures, and discomfort at the site of injection. Transient cutaneous or mucocutaneous reactions with or without fever, hypertension, and cardiac complications have also been identified (Cowgill, 1992).
Neutralizing Antibodies.
The problem of rHuEPO-related refractory anemia can be caused by the development of neutralizing anti-rHuEPO antibodies. The rHuEPO protein is antigenic in many dogs and cats, stimulating antibody titers as early as 4 weeks after treatment starts or many months later. Antibody titers decline after treatment stops, and attempts at immunosuppressive therapy to abrogate this response have not proved successful. It may be possible to test for antibody concentrations directly; as an alternative, bone marrow myeloid:erythroid ratios provide evidence of antibody formation. After rHuEPO is discontinued, pretreatment levels of erythropoiesis are attained (Polzin et al, 1995). However, such levels may not be sufficient to maintain adequate red cell numbers because severe anemia is present when rHuEPO is used.
Hypertension.
It has been hypothesized that patients with chronic anemia (as occurs with chronic renal failure) have peripheral vasodilation. This vasodilation may be corrected with rHuEPO therapy, resulting in hypertension caused by increased peripheral vascular resistance. Hypertension has been identified as a complication of therapy and does not appear to be the result of a direct pressor effect of the rHuEPO (Nissenson et al, 1991; Cowgill, 1992).
Blunted Response to rHuEPO.
Individual differences in response to rHuEPO are typical but not well understood. Several causes of blunted response or failure to resolve anemia have been identified in dogs and cats. These include antibodies to the rHuEPO in 20% to 30% of treated animals, functional or absolute iron deficiency, gastrointestinal loss of blood, hemolysis, and concurrent inflammatory or malignant diseases (Polzin et al, 1995).
WHEN TO INITIATE rHuEPO THERAPY.
The prevalence of anti-rHuEPO antibodies in treated dogs and cats makes the decision process regarding when to initiate therapy quite important. Most recommend using the drug only when definitely necessary because once an animal develops the antibodies, the drug no longer has any value to that individual. When the hematocrit values are below 25% in dogs or 20% in cats, anemia probably contributes to the adverse clinical signs associated with chronic renal failure. These PCV guidelines usually can be used to judge the severity of anemia; however, the degree of azotemia, expected progression rate of chronic renal failure, appetite, willingness to eat therapeutic diets, and progression rate of the anemia should all be considered in the risk-benefit analysis of when to start therapy (Polzin et al, 1995). Because many pet owners consider quality of life of prime importance, the advantages and disadvantages of rHuEPO treatment should be discussed with owners when anemia appears to be contributing to the dog’s or cat’s deteriorating quality of life.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree