Pamela A. Wilkins
Perinatal Asphyxia Syndrome
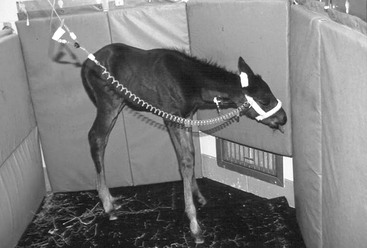
Hypoxic ischemic encephalopathy, also referred to as neonatal encephalopathy (NE), dummy syndrome, and neonatal maladjustment syndrome, is the most common clinical presentation of a broader syndrome of perinatal asphyxia syndrome (PAS). Although PAS is most clinically obvious as NE, the gastrointestinal tract and kidneys are frequently also affected, and complications associated with these systems should be expected. Cardiovascular, respiratory, and endocrine disorders may also be present. A wide spectrum of clinical signs is associated with NE, ranging from mild depression with loss of the suck reflex to grand mal seizure activity (Figure 175-1). Most affected foals are normal at birth but show signs of central nervous system abnormalities within a few hours following birth, whereas some will not show signs until 24 hours of age.
Predisposing Factors
Conditions associated with PAS in foals include dystocia, induced parturition, cesarean section, placentitis, premature placental separation, meconium aspiration, twin foals, fetal infection, severe maternal illness, maternal surgery, post-term pregnancies, and normal parturition. A fair number of foals have no known peripartum period of hypoxia. There is increasing evidence that cytokinemia, resulting from placental infection or insult, is a major contributor to NE in human infants.
Clinical Signs
Foals with PAS and NE may appear healthy at birth but develop central nervous system abnormalities from within a few hours of birth to 1 to 2 days of age. Clinical signs are extremely variable and can include one or more of the following: weakness, mental depression, mild to severe seizure activity, tremors, and hypertonia. Other clinical signs, such as inability to find the udder, inappropriate nursing behavior, loss of suckle reflex, loss of affinity for the dam, loss of recognition of environment, abnormal vocalization, dysphagia, weak tongue tone, central blindness, irregular respiratory pattern, and proprioceptive deficits, may be encountered. It is again important to recognize that other organ systems are generally affected to varying degrees. Repetitive detailed examination of other systems is a requirement of management of foals with PAS and NE.
Differential diagnoses for PAS and NE include severe sepsis, meningitis, cerebral trauma, cerebral vascular accidents, and developmental abnormalities such as hydrocephalus.
Pathophysiology
The underlying pathophysiologic details of PAS and NE in the foal are unknown and likely multifactorial. Neuronal cell death and injury secondary to a primary hypoxic or anoxic event underlie many cases. A second wave of neuronal cell death occurs during the reperfusion phase, in part because of apoptosis, whereas additional secondary cell death is thought to be caused by the neurotoxicity of glutamate and aspartate. There is evidence that this excitotoxic cascade that evolves during NE and PAS extends over several days from the time of insult and is modifiable. The activation of the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors is suspected to play a role in NE and PAS. Magnesium blocks NMDA receptors, and clinical trials investigating the efficacy of magnesium treatment following hypoxia in infants are underway.
Treatment
Therapy for the various manifestations of PAS involves control of seizures, general cerebral support, correction of metabolic abnormalities, maintenance of normal arterial blood gas values, tissue perfusion and renal function, and treatment of gastrointestinal dysfunction. Prevention, recognition, and treatment of secondary infections and general supportive care are also very important. It is important that seizures be controlled because cerebral oxygen consumption increases five-fold during seizures (Table 175-1). Diazepam and midazolam can be used for emergency control of seizures. If seizures are not readily stopped with these two agents, or if more than two seizures are recognized, diazepam should be replaced with either phenobarbital given to effect or a midazolam constant-rate infusion (CRI). The long half-life of phenobarbital in the foal (>200 hours) should be kept in mind when neurologic function is monitored after phenobarbital administration. Midazolam may be preferable to phenobarbital because it can be administered to effect and has fewer adverse effects; however, use of midazolam requires maintenance of a CRI. Foals with severe seizures may require a combination of these antiseizure medications. In foals with NE, ketamine and xylazine should be avoided because they can be associated with increased intracranial pressure.
TABLE 175-1
Drugs and Dosages Used in the Treatment of Perinatal Asphyxia Syndrome and Neonatal Encephalopathy
| Drug | Indication | Dose | Potential Complications |
| Diazepam | Single or short-term seizure control | 0.1-0.2 mg/kg (5-10 mg to a 50-kg foal IM or IV) | Rapid administration IV may result in respiratory depression. |
| Midazolam | Single or short-term seizure control | 0.1-0.2 mg/kg (5-10 mg to a 50-kg foal IM or IV) | Rapid administration IV may result in respiratory depression. |
| Midazolam CRI | Longer term control for repetitive seizures Mild sedation for hyperresponsive or jittery foals | 3-6 mg/hr Midazolam is water soluble, and CRI is administered in isotonic crystalloid fluid using a 0.5-mg/mL concentration. | Rapid administration IV may result in respiratory depression. Higher doses may be used if necessary. Advantage is ability to titrate to effect and reversibility if needed. |
| Phenobarbital | Longer term seizure control | 2-5 mg/kg IV slowly over 20 min. Start with lower dose and monitor to effect. Maximal effect expected after 45 min. | Respiratory depression, hypothermia, hypotension, and pharyngeal collapse, particularly at higher doses or in more severely affected foals |
| Thiamine | Metabolic support | 1-20 mg/kg q12 hr added to IV fluids (protect from light) | None |
| Mannitol | Intercellular edema: osmotic diuretic | 0.25-1.0 g/kg IV as a 20% solution rapidly over 15-20 min | Dehydration. May result in significant hyperosmolarity with repeated administration. May exacerbate cerebral bleeding |
| Dimethylsulfoxide | Antiinflammatory, intercellular edema: osmotic agent | 0.1-1 g/kg IV as a 10% solution | Odor; OSHA restrictions in some areas, hemolysis; dehydration |
| Gabapentin | Neuroprotection: GABA receptor agonist | 10-15 mg/kg/day divided equally and given orally 3 to 4 times a day | None described: an uninvestigated drug in equine neonates |
| Magnesium CRI* | Neuroprotection: NMDA receptor antagonist | Can precipitate other infusates; test compatibility or discontinue when administering intravenous antimicrobial drugs. | In very high doses (>10×), muscular weakness can occur, as can hypotension. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree